HC Verma Physics books are the most preferred books among students of CBSE schools. Students can be found referring to the chapters as well as practice questions at the end of each of these chapters, in the books. Students follow these textbooks religiously since quite a few questions in these also appear in exams.
Contents
- 1 HC Verma Solutions for Class 12 Physics Chapter 44 – X-rays
- 1.0.1 Page No 393:
- 1.0.2 Question 1:
- 1.0.3 Answer:
- 1.0.4 Question 2:
- 1.0.5 Answer:
- 1.0.6 Question 3:
- 1.0.7 Answer:
- 1.0.8 Question 4:
- 1.0.9 Answer:
- 1.0.10 Question 5:
- 1.0.11 Answer:
- 1.0.12 Question 6:
- 1.0.13 Answer:
- 1.0.14 Question 7:
- 1.0.15 Answer:
- 1.0.16 Question 8:
- 1.0.17 Answer:
- 1.0.18 Question 9:
- 1.0.19 Answer:
- 1.0.20 Question 10:
- 1.0.21 Answer:
- 1.0.22 Question 1:
- 1.0.23 Answer:
- 1.0.24 Question 2:
- 1.0.25 Answer:
- 1.0.26 Question 3:
- 1.0.27 Answer:
- 1.0.28 Question 4:
- 1.0.29 Answer:
- 1.0.30 Page No 394:
- 1.0.31 Question 5:
- 1.0.32 Answer:
- 1.0.33 Question 6:
- 1.0.34 Answer:
- 1.0.35 Question 7:
- 1.0.36 Answer:
- 1.0.37 Question 8:
- 1.0.38 Answer:
- 1.0.39 Question 9:
- 1.0.40 Answer:
- 1.0.41 Question 10:
- 1.0.42 Answer:
- 1.0.43 Question 11:
- 1.0.44 Answer:
- 1.0.45 Question 12:
- 1.0.46 Answer:
- 1.0.47 Question 13:
- 1.0.48 Answer:
- 1.0.49 Question 14:
- 1.0.50 Answer:
- 1.0.51 Question 1:
- 1.0.52 Answer:
- 1.0.53 Question 2:
- 1.0.54 Answer:
- 1.0.55 Question 3:
- 1.0.56 Answer:
- 1.0.57 Question 4:
- 1.0.58 Answer:
- 1.0.59 Question 5:
- 1.0.60 Answer:
- 1.0.61 Question 6:
- 1.0.62 Answer:
- 1.0.63 Page No 395:
- 1.0.64 Question 7:
- 1.0.65 Answer:
- 1.0.66 Question 8:
- 1.0.67 Answer:
- 1.0.68 Question 1:
- 1.0.69 Answer:
- 1.0.70 Question 2:
- 1.0.71 Answer:
- 1.0.72 Question 3:
- 1.0.73 Answer:
- 1.0.74 Question 4:
- 1.0.75 Answer:
- 1.0.76 Question 5:
- 1.0.77 Answer:
- 1.0.78 Question 6:
- 1.0.79 Answer:
- 1.0.80 Question 7:
- 1.0.81 Answer:
- 1.0.82 Question 8:
- 1.0.83 Answer:
- 1.0.84 Question 9:
- 1.0.85 Answer:
- 1.0.86 Question 10:
- 1.0.87 Answer:
- 1.0.88 Question 11:
- 1.0.89 Answer:
- 1.0.90 Question 13:
- 1.0.91 Answer:
- 1.0.92 Question 14:
- 1.0.93 Answer:
- 1.0.94 Question 15:
- 1.0.95 Answer:
- 1.0.96 Question 16:
- 1.0.97 Answer:
- 1.0.98 Question 17:
- 1.0.99 Answer:
- 1.0.100 Question 18:
- 1.0.101 Answer:
- 1.0.102 Question 19:
- 1.0.103 Answer:
- 1.0.104 Question 20:
- 1.0.105 Answer:
- 1.0.106 Question 21:
- 1.0.107 Answer:
- 1.0.108 Question 22:
- 1.0.109 Answer:
- 1.0.110 Question 23:
- 1.0.111 Answer:
- 1.0.112 Question 12:
- 1.0.113 Answer:
- 1.0.114 Page No 396:
- 1.0.115 Question 24:
- 1.0.116 Answer:
- 1.0.117 Question 25:
- 1.0.118 Answer:
- 1.0.119 Question 26:
- 1.0.120 Answer:
- 1.0.121 Question 27:
- 1.0.122 Answer:
- 2 Chapterwise HC Verma Solutions Class 12 Physics :
- 3 About the Author – HC Verma
HC Verma Solutions for Class 12 Physics Chapter 44 – X-rays
For such popular books, students can get extremely helpful practice material online. For all the questions in the HC Verma books, there are several sources where students can get detailed solutions and solve their doubts and queries.
Please note that these solutions are provided here for free.
Page No 393:
Question 1:
When a Coolidge tube is operated for some time it becomes hot. Where does the heat come from?
Answer:
A Coolidge tube apparatus consists of a filament and a target. The filament is heated to produce electrons that are accelerated by applying an electric field between the filament and the target. When these accelerated electrons enter the target, they collide with the target atoms. In the process, the electrons lose their kinetic energy. A part of this kinetic energy is utilised for emitting X-rays and the remaining energy is absorbed by the target. Inside the target, the kinetic energy of the electrons is converted into heat energy. This raises the temperature of the target and hence, it heats the Coolidge tube.
Question 2:
In a Coolidge tube, electrons strike the target and stop inside it. Does the target get more and more negatively charged as time passes?
Answer:
An electron emitted from the filament undergoes a number of collisions inside the material and loses its kinetic energy before coming to rest. This energy is utilised to give out photons or eject electrons from the atoms of the target. These electrons move to the battery connected to the circuit. Thus, the target does not get more and more negatively charged as time passes.
Question 3:
Can X-rays be used for photoelectric effect?
Answer:
Yes, X-rays can be use for photoelectric effect. Photoelectric effect is the emission of electrons from a metal surface when the frequency of radiation is greater than the threshold frequency of the metal. For photoelectric effect using X-rays, the energy of the incoming X-ray photon should be greater than the work-function of the metal used.
Question 4:
Can X-rays be polarised?
Answer:
Only transverse waves can be polarised. Since an X-ray is a transverse wave, it can be polarised.
Question 5:
X-ray and visible light travel at the same speed in vacuum. Do they travel at the same speed in glass?
Answer:
Speed of light in any material medium is inversely proportional to the refractive index of the medium. Since refractive index of glass for X-ray is less than that for visible light, an X-ray will travel at a faster speed than visible light in glass.
Question 6:
Characteristic X-rays may be used to identify the element from which they are being emitted. Can continuous X-rays be used for this purpose?
Answer:
Characteristic X-rays are emitted due to the transitions of electrons among different shells. The wavelength of the X-rays emitted in these transitions have definite value for a particular element. But continuous X-rays are emitted due to the conversion of kinetic energy of an electron into photon, which varies from collision to collision and is independent of material. Hence, continuous X-rays provide no information about the element from which they are being emitted.
Question 7:
Is it possible that in a Coolidge tube characteristic Lα X-rays are emitted but not Kα X-rays?
Answer:
Kα X-rays are emitted due to the transition of an electron from the L shell to the K shell and Lα X-rays due to the transition of an electron from the M shell to the L shell. If Kα X-rays are not emitted, then the L shell will not be vacant to take the electron from the M shell. Hence, Lα X-rays will not be emitted. Therefore, it is not possible that in a Coolidge tube, characteristic Lα X-rays are emitted but not Kα X-rays.
Question 8:
Can Lα X-ray of one material have shorter wavelength than Kα X-ray of another?
Answer:
An Lα X-ray is emitted when an electron jumps from the M to the L shell, and a Kα X-ray is emitted when an electron jumps from the L to the K shell. Less energy is involved when an electron jumps from the M to the L shell than when it jumps from the L to the K shell. Also, wavelength of a photon is inversely related to its energy. Therefore, an Lα X-ray has higher wavelength than a Kα X-ray for the same material.
Question 9:
Can a hydrogen atom emit characteristic X-rays?
Answer:
The difference of energy levels in a hydrogen atom is small. Hence, it is not able to emit characteristic X-rays.
Question 10:
Why is exposure to X-rays injurious to health but not exposure to visible light, when both are electromagnetic waves?
Answer:
X-rays have more penetrating power compared to visible light. As a result, they can penetrate the human body and can also damage the cells of the body. Prolonged exposure to X-rays can lead to cancer or genetic defects.
Question 1:
An X-ray beam can be deflected
(a) by an electric field
(b) by a magnetic field
(c) by an electric field as well as a magnetic field
(d) neither by an electric field nor a magnetic field
Answer:
(d) neither by an electric field nor a magnetic field
Since X-rays do not have any charged particles, they are not deflected by an electric field or a magnetic field.
Question 2:
Consider a photon of a continuous X-ray coming from a Coolidge tube. Its energy comes from
(a) the kinetic energy of the striking electron
(b) the kinetic energy of the free electrons of the target
(c) the kinetic energy of the ions of the target
(d) an atomic transition in the target
Answer:
(a) the kinetic energy of the striking electron
In an X-ray tube, electrons are emitted by the filament when it is heated. An electric field generated by a DC battery between the filament and the target makes the electrons hit the target atoms with a very high speed. As a result, the electrons lose their kinetic energy to eject photons, which leads to a continuous emission of X-rays.
Question 3:
The energy of a photon of a characteristic X-ray from a Coolidge tube comes from
(a) the kinetic energy of the striking electron
(b) the kinetic energy of the free electrons of the target
(c) the kinetic energy of the ions of the target
(d) an atomic transition in the target
Answer:
(d) an atomic transition in the target
In an X-ray tube, electrons are emitted by the filament. These electrons are made to strike the filament by applying an electric field between the filament and the target. As a result of it, the kinetic energy of the electrons is lost to the target atoms. This energy is utilised by the target atoms to knock out an electron from the innermost shell. Consequently, the electron makes a transition from the higher energy state to this vacant shell. Due to this transition, the difference of energy of the two states gives photon of characteristic X-ray.
Question 4:
If the potential difference applied to the tube is doubled and the separation between the filament and the target is also doubled, the cutoff wavelength
(a) will remain unchanged
(b) will be doubled
(c) will be halved
(d) will become four times the original
Answer:
(c) will be halved
Cut off wavelength is given by
λmin= hceV,
where h = Planck’s constant
c = speed of light
e = charge on an electron
V = potential difference applied to the tube
When potential difference (V) applied to the tube is doubled, cutoff wavelength
λ’minis given by
λ’min= hce2V
⇒λ’min=λmin2Cuttoff wavelength does not depend on the separation between the filament and the target.
Thus, cutoff wavelength will be halved if the the potential difference applied to the tube is doubled.
Page No 394:
Question 5:
If the current in the circuit for heating the filament is increased, the cutoff wavelength
(a) will increase
(b) will decrease
(c) will remain unchanged
(d) will change
Answer:
(c) will remain unchanged
Cut off wavelength is given by
λmin= hceV,
where h = Planck’s constant
c = speed of light
e = charge on an electron
V = potential difference applied to the tube
Clearly, the cutoff wavelength does not depend on the current in the circuit and temperature of the filament.
Question 6:
Moseley’s Law for characteristic X-ray is √v = a(Z − b). Here,
(a) both a and b are independent of the material
(b) a is independent but b depends on the material
(c) b is independent but a depends on the material
(d) both a and b depend on the material
Answer:
(a) both a and b are independent of the material
Moseley’s Law for characteristic X-ray is √v = a(Z − b), where, a and b are constants independent of the material used.
Question 7:
Frequencies of Kα X-rays of different materials are measured. Which one of the graphs in the figure may represent the relation between the frequency v and the atomic number Z ?
Figure
Answer:
Using Moseley’s Law,
v=aZ-b,
where v = frequency of Kα X-ray
Z = atomic number
∴ v=a2Z-b2⇒ Z-b2=va2This is the equation of a parabola with some intercept on the axis, representing atomic number (Z). Hence, curve d represent this relation correctly.
Question 8:
The X-ray beam emerging from an X-ray tube
(a) is monochromatic
(b) has all wavelengths smaller than a certain maximum wavelength
(c) has all wavelengths greater than a certain minimum wavelength
(d) has all wavelengths lying between a minimum and a maximum wavelength
Answer:
(c) has all wavelengths greater than a certain minimum wavelength
The X-ray beam emerging from an X-ray tube consists of wavelengths greater than a certain minimum wavelength called cutoff wavelength.
Question 9:
One of the following wavelengths is absent and the rest are present in the X-rays coming from a Coolidge tube. Which one is the absent wavelength?
(a) 25 pm
(b) 50 pm
(c) 75 pm
(d) 100 pm
Answer:
(a) 25 pm
Cutoff wavelength (minimum) is absent in the X-rays coming from a Coolidge tube. Hence, the 25 pm wavelength is absent.
Question 10:
The figure shows the intensity-wavelength relations of X-rays coming from two different Coolidge tubes. The solid curve represents the relation for the tube A in which the potential difference between the target and the filament is VA and the atomic number of the target material is ZA. These quantities are VB and ZB for the other tube. Then,
(a) VA > VB, ZA > ZB
(b) VA > VB, ZA < ZB
(c) VA < VB, ZA > ZB
(d) VA > VB, ZA < ZB
Figure
Answer:
(b) VA > VB, ZA < ZB
It is clear from the figure that the X-ray of tube A has less cutoff wavelength than the X-ray of tube B.
∴ λA<λBUsing Moseley’s Law,
ZA<ZB
λ∝1V, where V is the voltage applied in the X-ray tube.
∴ VA>VB
Question 11:
50% of the X-rays coming from a Coolidge tube are able to pass through a 0.1 mm thick aluminium foil. If the potential difference between the target and the filament is increased, the fraction of the X-rays passing through the same foil will be
(a) 0%
(b) < 50%
(c) 50 %
(d) > 50%
Answer:
(d) > 50%
The penetrating power of X-rays varies directly with the accelerating potential of the electrons (V) or the energy of the X-rays. So, if the potential difference between the target and the filament is increased, the fraction of the X-rays passing through the foil will also increase.
Question 12:
50% of the X-ray coming from a Coolidge tube is able to pass through a 0.1 mm thick aluminium foil. The potential difference between the target and the filament is increased. The thickness of the aluminium foil that will allow 50% of the X-ray to pass through will be
(a) zero
(b) < 0.1 mm
(c) 0.1 mm
d) > 0.1 mm
Answer:
(d) > 0.1 mm
As we increase the accelerating potential between the filament and the target, the penetrating power of the X-ray will increase. As a result, the fraction of the X-ray passing through the foil will increase. But it is given that the same fraction of the X-ray is passing. Therefore, the thickness of the aluminium foil should be increased to maintain the same fraction of the X-ray that will pass through the foil.
Question 13:
X-ray from a Coolidge tube is incident on a thin aluminium foil. The intensity of the X-ray transmitted by the foil is found to be I0. The heating current is increased to increase the temperature of the filament. The intensity of the X-ray transmitted by the foil will be
(a) zero
(b) < I0
(c) I0
(d) > I0
Answer:
(d) > I0.
We know that the intensity of an X-ray is directly proportional to the current through the X-ray tube. If the filament current is increased to increase the temperature of the filament, more electrons will be emitted from the filament per unit time. As a result, current in the X-ray tube will increase and consequently, the intensity of the X-ray will also increase.
Question 14:
Visible light passing through a circular hole forms a diffraction disc of radius 0.1 mm on a screen. If an X-ray is passed through the same setup, the radius of the diffraction disc will be
(a) zero
(b) < 0.1 mm
(c) 0.1 mm
(d) > 0.1 m
Answer:
(b) < 0.1 mm
Radius of the diffraction disc is directly proportional to the wavelength of the light used for the given hole. We know that the wavelength of an X-ray is less than the wavelength of visible light. If an X-ray is passed through the same setup, the radius of the diffraction disc will be less than 0.1 m.
Question 1:
For harder X-rays,
(a) the wavelength is higher
(b) the intensity is higher
(c) the frequency is higher
(d) the photon energy is higher.
Answer:
(c) the frequency is higher
(d) the photon energy is higher
Harder X-rays are the X-rays having low wavelengths. Since the frequency varies inversely with the wavelength, hard X-rays have high frequency.
Energy of a photon
Eis given by
E=hcλHere,
h = Planck’s constant
c = Speed of light
λ= Wavelength of light.
Clearly, energy varies inversely with wavelength. Therefore, the energy of the photon will be higher for the hard X-ray.
Question 2:
Cutoff wavelength of X-rays coming from a Coolidge tube depends on the
(a) target material
(b) accelerating voltage
(c) separation between the target and the filament
(d) temperature of the filament.
Answer:
(b) accelerating voltage
Cutoff wavelength
λminis given by
λmin = hceVHere,
h = Planck’s constant
c = Speed of light
V = Accelerating voltage
e = Charge of electron
Clearly, a cutoff wavelength depends on accelerating voltage. It does not depend on the target material, separation between the target and the temperature of the filament.
Question 3:
Mark the correct options.
(a) An atom with a vacancy has smaller energy that a neutral atom.
(b) K X-ray is emitted when a hole makes a jump from the K shell to some other shell.
(c) The wavelength of K X-ray is smaller than the wavelength of L X-ray of the same material.
(d) The wavelength of Kα X-ray is smaller than the wavelength of Kβ X-ray of the same material.
Answer:
(b) K X-ray is emitted when a hole makes a jump from the K shell to some other shell.
(c) The wavelength of K X-ray is smaller than the wavelength of L X-ray of the same material.
Energy of a vacant atom is higher than that of a neutral atom.
Hence, option (a) is incorrect.
K X-ray is emitted when an electron makes a jump to the K shell from some other shell. As a result, a positive charge hole is created in the outer shell. As the electron continuously moves to the K shell, the hole moves from the K shell to some other shell. Hence, option (b) is correct.
K X-ray is emitted due to the transition of an electron from the L or M shell to the K shell and L X-ray is emitted due to the transition of an electron from the M or N shell to the L shell. The energy involved in the transition from the L or M shell to the K shell is higher than the energy involved in the transition from the M or N shell to the L shell. Since the energy is inversely proportional to the wavelength, the wavelength of the K X-ray is smaller than the wavelength of the L X-ray of the same material. Hence, option (c) is correct.
If EK, EL and EM are the energies of K, L and M shells, respectively, then the wavelength of Kα X-ray
λ1is given by
λ1=hcEK-ELHere,
h = Planck’s constant
c = Speed of light
Wavelength of the Kβ X-ray
λ2is given by
λ2=hcEK-EM
As the difference of energies (EK
– EM) is more than (EK
–EL),
λ2is less than
λ1. Hence, option (d) is not correct.
Question 4:
For a given material, the energy and wavelength of characteristic X-rays satisfy
(a) E(Kα) > E(Kβ) > E(Kγ)
(b) E(Mα) > E(Lα) > E(Kα)
(c) λ(Kα) > λ(Kβ) > λ(Kγ)
(d) λ(Mα) > λ(Lα) > λ(Kα).
Answer:
(c) λ(Kα) > λ(Kβ) > λ(Kγ)
(d) λ(Mα) > λ(Lα) > λ(Kα)
The Kγ transition (from the N shell to the K shell) involves more energy than the Kβ transition (from the M shell to the K shell), which has more energy than the Kα transition (from the L shell to the K shell).
As the energy varies inversely with the wavelength,
λ(Kα) > λ(Kβ) > λ(Kγ)
The Mα transition is due to the jumping of an electron from the N shell to the M shell and involves less energy than the Lα transition (from the M shell to the L shell), which involves less energy than the Kα transition (from the L shell to the K shell).
As the energy varies inversely with the wavelength,
λ(Mα) > λ(Lα) > λ(Kα)
Question 5:
The potential difference applied to an X-ray tube is increased. As a result, in the emitted radiation,
(a) the intensity increases
(b) the minimum wavelength increases
(c) the intensity remains unchanged
(d) the minimum wavelength decreases.
Answer:
(c) the intensity remains unchanged
(d) the minimum wavelength decreases
Cutoff wavelength
λminis given by
λmin = hceVHere,
h = Planck’s constant
c = Speed of light
V = Accelerating voltage
e = Charge of electron
If the potential difference applied to an X-ray tube
Vis increased, then the minimum wavelength (cutoff wavelength) gets decreased.
Intensity is not affected by the potential difference applied.
Question 6:
When an electron strikes the target in a Coolidge tube, its entire kinetic energy
(a) is converted into a photon
(b) may be converted into a photon
(c) is converted into heat
(d) may be converted into heat.
Answer:
(b) may be converted into a photon
(d) may be converted into heat
When an electron strikes the target in a Coolidge tube, the kinetic energy of the electron is used in two ways. Some part of the kinetic energy is converted into a photon, while the remaining part gets converted into heat when the electron makes collisions with the atoms of the target. However, the amount of kinetic energy appearing as the photon vary from collision to collision.
Page No 395:
Question 7:
X-ray incident on a material
(a) exerts a force on it
(b) transfers energy to it
(c) transfers momentum to it
(d) transfers impulse to it.
Answer:
(a) exerts a force on it
(b) transfers energy to it
(c) transfers momentum to it
(d) transfers impulse to it.
An X-ray exerts force on the material on which it is incident. For example, it can penetrate into metals. An X-ray also transfers energy, momentum and impulse to the material on which it incident. Therefore, all options are correct.
Question 8:
Consider a photon of continuous X-ray and a photon of characteristic X-ray of the same wavelength. Which of the following is/are different for the two photons?
(a) Frequency
(b) Energy
(c) Penetrating power
(d) Method of creation
Answer:
(d) Method of creation
Let
λ1and
λ2be the wavelengths of the photons of continuous and characteristic X-rays, respectively.
Given:
λ1=λ2=λNow, frequency
νis given by
v=cλ1=cλ2⇒ν=cλHence, the frequency of both the photons is the same.
Energy of a photon
Eis given by
E=hvAs the frequency of both the photons is the same, they will have the same energy.
The photon of the continuous X-ray and the photon of the characteristic X-ray consist of the same penetrating power.
The photon of the characteristic X-ray is created because of the transition of an electron from one shell to another. The photon of the continuous X-ray is created because of the conversion of the kinetic energy of an electron into a photon of electromagnetic radiation.
Question 1:
Find the energy, the frequency and the momentum of an X-ray photon of wavelength 0.10 nm.
Answer:
Given:
Wavelength of the X-ray photon, λ = 0.1 nm
Speed of light, c = 3
×108 m/s
(a) Energy of the photon
Eis given by
E=hcλ⇒E=1242 eV 0.1 ⇒E=12420 eV⇒E=12.42 keV≈12.4 keV(b) Frequency is given by
ν=cλ⇒ν=3×1080.1×10-9⇒ν=3×1018 Hz(c) Momentum of the photon
Pis given by
P=Ec⇒P=12.4×103×1.6×10-193×108⇒P=6.613×10-24 kg-m/s≈6.62×10-24 g-m/s
Question 2:
Iron emits Kα X-ray of energy 6.4 keV. Calculate the times taken by an iron Kα photon to cross through a distance of 3 km.
Answer:
Given:
Energy of the X-ray, E = 6.4 keV
Distance travelled by the photon, d = 3 km = 3
×103 m
Time taken by the photon to cross the distance of 3 km is given by
t=DistanceSpeed=3×1033×108t=10-5 s=10×10-6 st=10 μsBoth the Kα photon and X-ray will take the same time, that is, 10
μs, to cross the distance of 3 km.
Question 3:
Find the cutoff wavelength for the continuous X-rays coming from an X-ray tube operating at 30 kV.
Answer:
Given:
Voltage of the X-ray tube, V = 30 kV
Cutoff wavelength for continuous X-rays
λis given by
λ=hceVHere, h=Planck’s constantV=Voltage of the X-ray tubec=Speed of light∴λ=1242 eV-nme×30×103⇒λ=414×10-4 nm=41.4 pm
Question 4:
What potential difference should be applied across an X-ray tube to get X-ray of wavelength not less than 0.10 nm? What is the maximum energy of a photon of this X-ray in joule?
Answer:
Given:
Wavelength of the X-ray,
λ= 0.10 nm
Planck’s constant, h = 6.63
×10
-34J-s
Speed of light, c = 3
×108m/s
Minimum wavelength is given by
λmin=hceV⇒V=hceλmin⇒V=6.63×10-34×3×1081.6×10-19×10-10⇒V=12.43×103 V=12.4 kVMaximum energy of the photon
Eis given by
E =
hcλ
⇒E=6.68×10-34×3×10810-10⇒E=19.89×10-16⇒E=1.989×10-15≈2×10-15 J
Question 5:
The X-ray coming from a Coolidge tube has a cutoff wavelength of 80 pm. Find the kinetic energy of the electrons hitting the target.
Answer:
Given:
Cutoff wavelength of the Coolidge tube,
λ= 80 pm
Energy of the electron hitting the target
Eis given by
E=hcλHere,
h = Planck’s constant
c = Speed of light
λ= Wavelength of light
∴ E =1242 eV-nm80×10-3⇒E=1242×10-9 eV80×10-12⇒E=15.525×103 eV≈15.5 keV
Question 6:
If the operating potential in an X-ray tube is increased by 1%, by what percentage does the cutoff wavelength decrease?
Answer:
Let
λbe the cut off wavelength and V be the operating potential in the X-ray tube.
Then,
λ=hcVHere,
h = Planck’s constant
c = Speed of light
If the operating voltage is increased by 1%, then the new operating voltage (V‘) will be given by
V’=V+1100×VV’= 1.01 VCut off wavelength
λ’on increasing the operating voltage is given by
λ’=hc1.01V=λ1.01∴ λ’-λ=0.011.01λPercentage change in the wavelength is given by
0.01λ1.01×λ×100=11.01=0.9901 = 1 (approx.)
Question 7:
The distance between the cathode (filament) and the target in an X-ray tube is 1.5 m. If the cutoff wavelength is 30 pm, find the electric field between the cathode and the target.
Answer:
Given:
Distance between the filament and the target in the X-ray tube, d = 1.5 m
Cut off wavelength,
λ= 30 pm
Energy
Eis given by
E=hcλHere,
h = Planck’s constant
c = Speed of light
λ= Wavelength of light
Thus, we have
E=1242 eV-nm30×10-3⇒E=1242×10-930×10-12⇒E=41.4×103 eVNow,Electric field =Vd =41.4×1031.5 =27.6×103 V/m =27.6 kV/m
Question 8:
The short-wavelength limit shifts by 26 pm when the operating voltage in an X-ray tube is increased to 1.5 times the original value. What was the original value of the operating voltage?
Answer:
Let
λbe the initial wavelength, V be the initial potential,
λ’be the new wavelength and V‘ be the new operating voltage when the operating voltage is increased in the X-ray tube.
Given:
λ’=
λ -26 pm
V‘ = 1.5 V
Energy
Eis given by
E=hcλ
⇒eV=hcλHere,
h = Planck’s constant
c = Speed of light
λ= Wavelength of light
V = Operating potential
∴λ=hceV ⇒λV=λ’V’ ∵λ∝1V⇒λV=λ-26×1.5 V⇒ 0.5λ=26×1.5⇒λ=26×3⇒λ=78 pmHence, the initial wavelength is 78
×10
-12 m.
Now, the operating voltage (V) is given by
V=hceλ⇒V=6.63×10-34×3×1081.6×10-19×78×10-12⇒V=0.15937×105⇒V=15.9 kV
Question 9:
The electron beam in a colour TV is accelerated through 32 kV and then strikes the screen. What is the wavelength of the most energetic X-ray photon?
Answer:
Given:
Potential of the electron beam, V = 32 kV = 32 × 103 V
Energy,
E=32×103 eVWavelength of the X-ray photon
λis given by
λ=hcEHere,
h = Planck’s constant
c = Speed of light
∴λ=hcE⇒λ=1242 eVnm32×103⇒λ=38.8×10-3 nm⇒λ=38.8 pm
Question 10:
When 40 kV is applied across an X-ray tube, X-ray is obtained with a maximum frequency of 9.7 × 1018 Hz. Calculate the value of Planck constant from these data.
Answer:
Given:
Potential applied to the X-ray tube, V = 40 kV
Frequency of the X-ray, v = 9.7 × 1018 Hz
Wavelength
λis given by
λ=hceVHere,
h = Planck’s constant
c = Speed of light
e = 1.6
×10
-19
∴λ=hceV⇒ λc=heV ⇒ 1v=heV ∵v=cλ⇒ h=eVv⇒h=40×1039.7×1018×e⇒h=4.12×10-15 eVs
Question 11:
An X-ray tube operates at 40 kV. Suppose the electron converts 70% of its energy into a photon at each collision. Find the lowest there wavelengths emitted from the tube. Neglect the energy imparted to the atom with which the electron collides.
Answer:
Given:
Potential of the X-ray tube, V = 40 kV = 40 × 103 V
Energy = 40 × 103 eV
Energy utilised by the electron is given by
E =
70100×40×103= 28
×103 eV
Wavelength
λis given by
λ=hcEHere,
h = Planck’s constant
c = Speed of light
E = Energy of the electron
∴λ=hcE⇒λ=1242 eV-nm28×103 eV⇒λ=1242×10-9 eV28×103 eV⇒λ=44.35×10-12⇒λ=44.35 pmFor the second wavelength,
E = 70% (Leftover energy)
=70100×(40-28)103=70100×12×103=84×102 eVAnd,λ=hcE=12428.4×103=147.86×10-3 nm=147.86 pm=148 pmFor the third wavelength,
E=70100(12-8.4)×103 =7×3.6×102=25.2×102 eVAnd,λ=hcE=124225.2×102 =49.2857×10-2 =493 pm
Question 13:
The Kβ X-ray of argon has a wavelength of 0.36 nm. The minimum energy needed to ionize an argon atom is 16 eV. Find the energy needed to knock out an electron from the K shell of an argon atom.
Answer:
Given:
Wavelength of Kβ X-ray of argon, λ = 0.36 nm
Energy needed to ionise an argon atom = 16 eV
Energy of Kβ X-ray of argon
Eis given by
E=12420.36=3450 eVEnergy needed to knock out an electron from K shell
EK = (3450 + 16) eV
EK = 3466 eV
≈3.47 keV
Question 14:
The Kα X-rays of aluminium (Z = 13) and zinc (Z = 30) have wavelengths 887 pm and 146 pm respectively. Use Moseley’s law √v = a(Z − b) to find the wavelengths of the Kα X-ray of iron (Z = 26).
Answer:
Given:
Wavelength of Kα X-rays of aluminium, λ1 = 887 pm
Frequency of X-rays of aluminium is given by
νa=cλνa=3×108887×10-12νa=3.382×1017νa=33.82×1016 HzWavelength of Kα X-rays of zinc,
λ2= 146 pm
Frequency of X-rays of zinc is given by
νz=3×108146×10-12νz=0.02055×1020νz=2.055×1018 HzWe know
ν= a(Z − b)
For aluminium,
5.815 × 108 = a(13 − b) …(1)
For zinc,
1.4331 × 109 = a(30 − b) …(2)
Dividing (1) by (2)
13-b30-b=5.815×10-11.4331=0.4057⇒ 30×0.4057-0.4057 b=13-b⇒ 12.171-0.4057 b+b=13b=0.8290.5943=1.39491a=5.815×10811.33=0.51323×108=5×107For Fe,
Frequency
ν’is given by
ν’ = 5× 107 (26 − 1.39)
= 5 × 24.61 × 107
= 123.05 × 107
ν’ =
cλHere, c = speed of light
λ= Wavelength of light
∴ cλ=5141.3×1014⇒λ=3×1085141.3×1014 =0.000198×10-5 m =198×10-12=198 pm
Question 15:
A certain element emits Kα X-ray of energy 3.69 keV. Use the data from the previous problem to identify the element.
Answer:
Given:
Energy of Kα X-ray, E = 3.69 keV = 3690 eV
Wavelength
λis given by
λ=hcEλ=12423690λ=0.33658 nm⇒λ=0.34×10-9 m
From Moseley’s equation,
cλ=a(Z-b)Here, c = speed of light
λ= wavelength of light
Z = atomic number of element
On substituting the respective values,
3×1080.34×10-9=5×107(Z-1.39)⇒8.82×1017=5×107(Z-1.39)⇒ 9.39×108=5×107(Z-1.39)⇒93.95=Z-1.39⇒Z=93.95+1.39 =20.17=20So, the element is calcium.
Question 16:
The Kβ X-rays from certain elements are given below. Draw a Moseley-type plot of √v versus Z for Kβ radiation.
| Element | Ne | P | Ca | Mn | Zn | Br |
| Energy (keV) | 0.858 | 2.14 | 4.02 | 6.51 | 9.57 | 13.3 |
Answer:
We can directly get value of v by energy frequency relation.
hv = energy
Here, h = Planck’s constant
v=Energy (in keV)hThe required graph is as follows: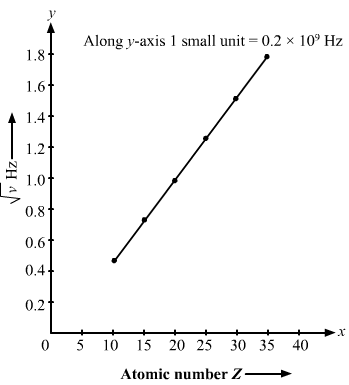
Question 17:
The Kα and Kβ X-rays of molybdenum have wavelengths 0.71 A and 0.63 A respectively. Find the wavelength of Lα X-ray of molybdenum.
Answer:
Given:
Wavelength of Kα X-rays,
λ1 = 0.71 A
Wavelength of Kβ X-rays,
λ2= 0.63 A
For La X-ray of molybdenum,
a = 57
b = 1
From Moseley’s equation,
v=a(Z-b)Here, v = frequency of X-ray
Z = atomic number of the element
v=a(Z-b)⇒v=a(57-1) =a×56 …(1)For Cu (29),1.88×1018=a(29-1)=28 a …(2)Dividing (1) and (2)
v1.88×1018=a×56a×28=2⇒ v=1.88×1018×(2)2 =4×1.88×1018 =7.52×1018 Hz
Question 18:
The Kα and Kβ X-rays of molybdenum have wavelengths 0.71 A and 0.63 A respectively. Find the wavelength of Lα X-ray of molybdenum.
Answer:
Given:
Wavelength of Kα X-rays of molybdenum,
λa= 0.71 A
Wavelength of Kβ X-rays of molybdenum,
λb= 0.63 A
Energy of KαX-rays
Kais given by
Ka = EK
–EL …..(1)
Energy of Kβ X-rays
Kβis given by
Kβ=EK-EM ….(2)
Energy of La X-ray
Lais given by
KL=EL-EMSubtracting (2) from (1),
Kα-Kβ=EM-EL=-KLor KL=Kβ-Kα KL=3×1080.63×10-10-3×1080.71×10-10 KL=4.761×10-18-4.225×1018 KL=0.536×1018 HzAgain, λ=3×1080.536×10-18 ⇒λ=5.6×10-10=5.6 Å
Question 19:
The wavelengths of Kα and Lα X-rays of a material are 21.3 pm and 141 pm respectively. Find the wavelength of Kβ X-ray of the material.
Answer:
Given:
Wavelength of Kα X-ray,
λ1= 21.3 pm
Wavelength of Lα X-ray,
λ2= 141 pm
Energy of Kα X-ray
E1is given by
E1=124221.3×10-3 =58.309×103 eVEnergy of Lα X-ray
E2is given by
E2=1242141×10-5 =8.8085×103 eVEnergy of Kβ X-ray
E3will be
E3=E1+E2E3=(58.309+8.809)×103 eV E3=67.118×103 eVWavelength of Kβ X-ray
λis given by
λ=hcE3=124267.118×103 =18.5×10-3 nm=18.5 pm
Question 20:
The energy of a silver atom with a vacancy in K shell is 25.31 keV, in L shell is 3.56 keV and in M shell is 0.530 keV higher than the energy of the atom with no vacancy. Find the frequency of Kα, Kβ and Lα X-rays of silver.
Answer:
Given:
Energy of electron in the K shell, Ek = 25.31 keV
Energy of electron in the L shell, EL = 3.56 keV
Energy of electron in the M shell, EM = 0.530 keV
Let f be the frequency of K
αX-ray and f0 be the frequency of Kβ X-ray.
Let f1 be the frequency of Lα X-rays of silver.
∴ Kα = EK − EL = hf
Here, h = Planck constant
f = frequency of K
αX-ray
f=EK-ELhf=(25.31-3.56)6.63×10-34×1.6×10-19×103f=21.75×103×10156.67f=5.25×1018 HzKβ=EK-EM=hf0⇒f0=EK-EMh⇒f0=(25.31-0.53)6.67×10-34×103×1.6×10-19⇒f0=5.985×1018 HzKL=EL-EM=hf1f1=EL-EMhf1= 3.56-0.5306.63×10-34×103×1.6×10-19f1= 7.32×1017 Hz
Question 21:
Find the maximum potential difference which may be applied across an X-ray tube with tungsten target without emitting any characteristic K or L X-ray. The energy levels of the tungsten atom with an electron knocked out are as follows.
| Cell containing vacancy | K | L | M |
| Energy in keV | 69.5 | 11.3 | 2.3 |
Answer:
Let the potential required that may be applied across the X-ray tube without emitting any characteristic K or L X-ray be V.
∴ Energy of electron = eV
This amount of energy is equal to the energy of L shell.
So, the maximum potential difference that can be applied without emitting any electron is 11.3 kV.
Question 22:
The electric current in an X-ray tube (from the target to the filament) operating at 40 kV is 10 mA. Assume that on an average, 1% of the total kinetic energy of the electron hitting hte target are converted into X-rays.
(a) What is the total power emitted as X-rays and (b) how much heat is produced in the target every second?
Answer:
Given:
V = 30 KV
i = 10mA
1% of TKE (total kinetic energy) = X-ray
i = ne
or n=10-21.6×10-19=0.625×1017 (number of electrons)
KE of one electron = eV
=1.6×10-19×40×103=6.4×10-15JTKE=0.625×6.4×1017×10-15=4×102J(b) Heat produced in target per second = 400 − 4 = 396 J
Question 23:
Heat at the rate of 200 W is produced in an X-ray tube operating at 20 kV. Find the current in the circuit. Assume that only a small fraction of the kinetic energy of electrons is converted into X-rays.
Answer:
Given:
Heat produced/second = Power (P) = 200 W
Potential in the X ray tube, V = 20 kV
We know
Power, P = VI
Here, V = potential difference
I = current
But I =
netWhere, e = charge on electron
t = time
n = no of electrons
∴ P=neVt⇒ 200 =neVt⇒ (ne/t)V=200⇒ I=200V⇒I=20020×103=10 mA
Question 12:
The wavelength of Kα X-ray of tungsten is 21.3 pm. It takes 11.3 keV to knock out an electron from the L shell of a tungsten atom. What should be the minimum accelerating voltage across an X-ray tube having tungsten target which allows production of Kα X-ray?
Answer:
Given:
Wavelength of X-ray of tungsten,
λ= 21.3 pm
Energy required to take out electron from the L shell of a tungsten atom, EL = 11.3 keV
Voltage required to take out electron from the L shell of a tungsten atom, VL = 11.3 kV
Let EK and EL be the energies of K and L, respectively.
EK-EL=hcλHere, h=Planck’s constant c=Speed of lightEK-EL=1242 eV-nm21.3×10-12EK-EL=1242×10-9 eV21.3×10-12EK-EL=58.309 keVEL=11.3 keV∴ EK=69.609 keVThus, the accelerating voltage across an X-ray tube that allows the production of Kα X-ray is given by
VK = 69.609 kV
Page No 396:
Question 24:
Continuous X-rays are made to strike a tissue paper soaked with polluted water. The incoming X-rays excite the atoms of the sample by knocking out the electrons from the inner shells. Characteristic X-rays are analysed and the intensity is plotted against the wavelength. Assuming that only Kα intensities are detected, list the elements present in the sample from the plot. Use Moseley’s equation v − (25 × 1014Hz)(Z − 1)2.
Figure
Answer:
Given:
f = (25 × 1014 Hz) (Z − 1)2
or cλ=25×1014(Z-1)2(a) 3×10878.9×10-12×25×1014=(Z-1)2or (Z-1)2=.0.001520×106=1520⇒ Z-1=38.98or Z=39.98=40Thus, it is (Zr).(b) 3×108146×10-12×25×1014=(Z-1)2or (Z-1)2=0.00082219×106or Z-1=28.669or Z=29.669=30Thus, it is (Zn).(c) 3×108158×10-12×25×104=(Z-1)2or (Z-1)2=0.0007594×104or Z-1=27.5559or Z=28.5589=29Thus, it is (Cu).(d) 3×108198×10-12×25×104=(Z-1)2or (Z-1)2=0.000606×104or Z-1=24.6162or Z=25.6162=26Thus, it is (Fe).
Question 25:
A free atom of iron emits Kα X-rays of energy 6.4 keV. Calculate the recoil kinetic energy of the atom. Mass of an iron atom = 9.3 × 10−26 kg.
Answer:
Here, energy of photon = E
E = 6.4 KeV = 6.4 × 103 eV
Momentum of photon
=Ec=6.4×1033×108=3.41×10-24kgm/secAccording to collision theory,
Momentum of photon = Momentum of atom
∴ Momentum of atom, P = 3.41 × 10−24 kgm/sec
Recoil KE of atom = P2/2M
=(3.41×10-24)2eV(20)9.3×10-26×1.6×10-19=3.9eV[1 Joule = 1.6 × 10−19 eV]
Question 26:
The stopping potential in a photoelectric experiment is linearly related to the inverse of the wavelength (1/λ) of the light falling on the cathode. The potential difference applied across an X-ray tube is linearly related to the inverse of the cutoff wavelength (1/λ) of the X-ray emitted. Show that the slopes of the lines in the two cases are equal and find its value.
Answer:
V0 – Stopping Potential
K – Potential difference across X-ray tube
λ – Wavelength
λ – Cut difference Wavelength
eV0=hf-hf0λ=hceVeV0=hcλor Vλ=hceor V0λ=hceHere, the slopes are same.
i.e. V0λ = Vλ
hce=6.63×10-34×3×1081.6×10-19=1.242×10-6 Vm
Question 27:
Suppose a monochromatic X-ray beam of wavelength 100 pm is sent through a Young’s double slit and the interference pattern is observed on a photographic plate placed 40 cm away from the slit. What should be the separation between the slits so that the successive maxima on the screen are separated by a distance of 0.1 mm?
Answer:
Given:
λ=10 pm=100×10-12mD=40 cm=40×10-2mβ=0.1 mm=0.1×10-3mβ=λDdd=λDβ=100×10-12×40×10-210-3×0.1=4×10-7m
Chapterwise HC Verma Solutions Class 12 Physics :
- Chapter 23 – Heat and Temperature
- Chapter 24 – Kinetic Theory of Gases
- Chapter 25 – Calorimetry
- Chapter 26 – Laws of Thermodynamics
- Chapter 27 – Specific Heat Capacities of Gases
- Chapter 28 – Heat Transfer
- Chapter 29 – Electric Field and Potential
- Chapter 30 – Gauss’s Law
- Chapter 31 – Capacitors
- Chapter 32 – Electric Current in Conductors
- Chapter 33 – Thermal and Chemical Effects of Electric Current
- Chapter 34 – Magnetic Field
- Chapter 35 – Magnetic Field due to a Current
- Chapter 36 – Permanent Magnets
- Chapter 37 – Magnetic Properties of Matter
- Chapter 38 – Electromagnetic Induction
- Chapter 39 – Alternating Current
- Chapter 40 – Electromagnetic Waves
- Chapter 41 – Electric Current through Gases
- Chapter 42 – Photoelectric Effect and Wave Particle Duality
- Chapter 43 – Bohr’s Model and Physics of the Atom
- Chapter 44 – X-rays
- Chapter 45 – Semiconductors and Semiconductor Devices
- Chapter 46 – The Nucleus
- Chapter 47 – The Special Theory of Relativity
About the Author – HC Verma
HC Verma, the author of many popular and well-renowned Physics books, was born on 8 April 1952. Passing out from one of the most prestigious colleges of the country, IIT Kanpur, he worked as an experimental physicist in the Department of Nuclear Physics.
His most famous works which he is known for include the two-volume Concepts of Physics. He also worked for the social upliftment of the economically weaker children through his organization named Shiksha Sopan. He is also the recipient of the Padma Shri, which is considered India’s fourth-highest civilian award. He received the same because of his contribution and valuable work in the field of Physics.
