HC Verma Physics books are the most preferred books among students of CBSE schools. Students can be found referring to the chapters as well as practice questions at the end of each of these chapters, in the books. Students follow these textbooks religiously since quite a few questions in these also appear in exams.
Contents
- 1 HC Verma Solutions for Class 12 Physics Chapter 42 – Photoelectric Effect and Wave Particle Duality
- 1.0.1 Page No 363:
- 1.0.2 Question 1:
- 1.0.3 Answer:
- 1.0.4 Question 2:
- 1.0.5 Answer:
- 1.0.6 Question 3:
- 1.0.7 Answer:
- 1.0.8 Question 4:
- 1.0.9 Answer:
- 1.0.10 Question 5:
- 1.0.11 Answer:
- 1.0.12 Question 6:
- 1.0.13 Answer:
- 1.0.14 Question 7:
- 1.0.15 Answer:
- 1.0.16 Question 8:
- 1.0.17 Answer:
- 1.0.18 Question 9:
- 1.0.19 Answer:
- 1.0.20 Question 10:
- 1.0.21 Answer:
- 1.0.22 Question 11:
- 1.0.23 Answer:
- 1.0.24 Question 12:
- 1.0.25 Answer:
- 1.0.26 Question 13:
- 1.0.27 Answer:
- 1.0.28 Question 1:
- 1.0.29 Answer:
- 1.0.30 Question 2:
- 1.0.31 Answer:
- 1.0.32 Question 3:
- 1.0.33 Answer:
- 1.0.34 Question 4:
- 1.0.35 Answer:
- 1.0.36 Question 5:
- 1.0.37 Answer:
- 1.0.38 Question 6:
- 1.0.39 Answer:
- 1.0.40 Question 7:
- 1.0.41 Answer:
- 1.0.42 Page No 364:
- 1.0.43 Question 8:
- 1.0.44 Answer:
- 1.0.45 Question 9:
- 1.0.46 Answer:
- 1.0.47 Question 10:
- 1.0.48 Answer:
- 1.0.49 Question 11:
- 1.0.50 Answer:
- 1.0.51 Question 12:
- 1.0.52 Answer:
- 1.0.53 Question 13:
- 1.0.54 Answer:
- 1.0.55 Question 14:
- 1.0.56 Answer:
- 1.0.57 Question 1:
- 1.0.58 Answer:
- 1.0.59 Question 2:
- 1.0.60 Answer:
- 1.0.61 Question 3:
- 1.0.62 Answer:
- 1.0.63 Question 4:
- 1.0.64 Answer:
- 1.0.65 Question 5:
- 1.0.66 Answer:
- 1.0.67 Question 6:
- 1.0.68 Answer:
- 1.0.69 Question 7:
- 1.0.70 Answer:
- 1.0.71 Page No 365:
- 1.0.72 Question 1:
- 1.0.73 Answer:
- 1.0.74 Question 2:
- 1.0.75 Answer:
- 1.0.76 Question 3:
- 1.0.77 Answer:
- 1.0.78 Question 4:
- 1.0.79 Answer:
- 1.0.80 Question 5:
- 1.0.81 Answer:
- 1.0.82 Question 6:
- 1.0.83 Answer:
- 1.0.84 Question 7:
- 1.0.85 Answer:
- 1.0.86 Question 8:
- 1.0.87 Answer:
- 1.0.88 Question 9:
- 1.0.89 Answer:
- 1.0.90 Question 10:
- 1.0.91 Answer:
- 1.0.92 Question 11:
- 1.0.93 Answer:
- 1.0.94 Question 12:
- 1.0.95 Answer:
- 1.0.96 Question 13:
- 1.0.97 Answer:
- 1.0.98 Question 14:
- 1.0.99 Answer:
- 1.0.100 Question 15:
- 1.0.101 Answer:
- 1.0.102 Question 16:
- 1.0.103 Answer:
- 1.0.104 Question 17:
- 1.0.105 Answer:
- 1.0.106 Question 18:
- 1.0.107 Answer:
- 1.0.108 Question 19:
- 1.0.109 Answer:
- 1.0.110 Question 20:
- 1.0.111 Answer:
- 1.0.112 Question 21:
- 1.0.113 Answer:
- 1.0.114 Page No 366:
- 1.0.115 Question 22:
- 1.0.116 Answer:
- 1.0.117 Question 23:
- 1.0.118 Answer:
- 1.0.119 Question 24:
- 1.0.120 Answer:
- 1.0.121 Question 25:
- 1.0.122 Answer:
- 1.0.123 Question 26:
- 1.0.124 Answer:
- 1.0.125 Question 27:
- 1.0.126 Answer:
- 1.0.127 Question 28:
- 1.0.128 Answer:
- 1.0.129 Question 29:
- 1.0.130 Answer:
- 1.0.131 Question 30:
- 1.0.132 Answer:
- 1.0.133 Question 31:
- 1.0.134 Answer:
- 1.0.135 Question 32:
- 1.0.136 Answer:
- 1.0.137 Question 33:
- 1.0.138 Answer:
- 1.0.139 Question 34:
- 1.0.140 Answer:
- 1.0.141 Question 35:
- 1.0.142 Answer:
- 2 Chapterwise HC Verma Solutions Class 12 Physics :
- 3 About the Author – HC Verma
HC Verma Solutions for Class 12 Physics Chapter 42 – Photoelectric Effect and Wave Particle Duality
For such popular books, students can get extremely helpful practice material online. For all the questions in the HC Verma books, there are several sources where students can get detailed solutions and solve their doubts and queries.
Please note that these solutions are provided here for free.
Page No 363:
Question 1:
Can we find the mass of a photon by the definition p = mv?
Answer:
No, we cannot find the mass of a photon by the definition p = mv. The equation p = mv is valid only for objects that move with a velocity that is much slower than the speed of light. The momentum of a relativistic particle like photon is given by
pc=E2-m2c4.
A photon has zero rest mass. Therefore, on putting m = 0 in the equation, we get
p=Ec, which is the valid equation for a photon.
Question 2:
Is it always true that for two sources of equal intensity, the number of photons emitted in a given time are equal?
Answer:
Let the source’s area be A, and intensity of the source be I. The energy of each emitted photonis E. Then, the number of photons emitted in a given time will be
n=IAE.
If the areas of the sources and the wavelengths of light emitted by the two sources are different, then the number of photons emitted will be different.
Question 3:
What is the speed of a photon with respect to another photon if (a) the two photons are going in the same direction and (b) they are going in opposite directions?
Answer:
(a) In relativity, the relative speed of two objects
vrelmoving in the same direction with speeds u and v is given by
vrel=u-v1-uvc2 …1 As the photons are moving with the speed of light, u = c and v = c.
On substituting the values of u and v in equation (1), we get:
vrel= 0
Thus, relative velocity of a photon with respect to another photon will be 0, when they are going in the same direction.
(b) In relativity, relative speed of two objects moving in opposite directions with speeds u and v is given by
vrel=u+v1+uvc2 …2 We know that a photon travels with the speed of light. Therefore, u = c and v = c
On substituting the values of u and v in equation (2), we get:
vrel=c Thus, the relative velocity of a photon with respect to another photon will be equal to the speed of light when they are going in opposite directions.
Question 4:
Can a photon be deflected by an electric field? Or by a magnetic field?
Answer:
Photons are electrically neutral. Hence, they are not deflected by electric and magnetic fields.
Question 5:
A hot body is placed in a closed room maintained at a lower temperature. Is the number of photons in the room increasing?
Answer:
As the hot body is placed in a closed room maintained at a lower temperature, there will be transfer of heat in the room through convection and radiation. Heat radiation also consists of photons; therefore, photons will be emitted by the hot body. Hence, the number of photons in the room will increase.
Question 6:
Should the energy of a photon be called its kinetic energy or its internal energy?
Answer:
Relativistic equation of energy:
E2=p2c2+m2c4 …1Here, p2c2 = kinetic energy of photon
m02c4 = internal energy of photon
We know photons have zero rest mass. Therefore, m0 = 0.
Substituting the value of m0 = 0 in equation (1), we get:
E=pcThus, the energy of a photon should be called its kinetic energy.
Question 7:
In an experiment on photoelectric effect, a photon is incident on an electron from one direction and the photoelectron is emitted almost in the opposite direction. Does this violate the principle of conservation of momentum?
Answer:
No, it does not violate the principle of conservation of momentum. In the photon-electron collision, the energy and momentum are conserved.
Question 8:
It is found that yellow light does not eject photoelectrons from a metal. Is it advisable to try with orange light or with green light?
Answer:
Photoelectrons are emitted from a metal’s surface if the frequency of incident radiation is more than the threshold frequency of the given metal surface. As yellow light does not eject photoelectrons from a metal it means that the threshold frequency of the metal is more than the frequency of yellow light. Since the frequency of orange light is less than the frequency of yellow light, therefore it will not be able to eject photoelectrons from the metal’s surface. The frequency of green light is more than the frequency of yellow light. Hence, when it is incident on the metal surface, it will eject electrons from the metal.
Question 9:
It is found that photosynthesis starts in certain plants when exposed to sunlight, but it does not start if the plants are exposed only to infrared light. Explain.
Answer:
Photosynthesis starts when a plant is exposed to visible light. The visible light’s photons possess just enough energy to excite the electrons of molecules of the plant without causing damage to its cells. Infrared rays have less frequency than visible light. Due to this, the energy of the photons of infrared rays are not sufficient to initiate photosynthesis. Therefore, photosynthesis does not start if plants are exposed only to infrared light.
Question 10:
The threshold wavelength of a metal is λ0. Light of wavelength slightly less than λ0 is incident on an insulated plate made of this metal. It is found that photoelectrons are emitted for some time and after that the emission stops. Explain.
Answer:
When light of wavelength less than λ0 is incident on the metal surface, the free electrons of the metal will gain energy and come out of the metal surface. As the metal plate is insulated (it is not connected with the battery), the free electrons of the metal will not be replaced by the other electrons. Hence, photoelectron emission will stop after some time.
Question 11:
Is p − E/c valid for electrons?
Answer:
From Einstein’s mass- energy equation,
E = mc2
⇒E=m0c21-v2c2Relativistic momentum,
p=mv⇒p=m0c21-v2c2Combining the above equations, we get:
E2=m02c4+p2c2From the above equation, it is clear that for p = E/c to be valid, the rest mass of the body should be zero. As electrons do not have zero rest mass, this equation is not valid for electrons.
Question 12:
Consider the de-Broglie wavelength of an electron and a proton. Which wavelength is smaller if the two particles have (a) the same speed (b) the same momentum (c) the same energy?
Answer:
de-Broglie wavelength,
λ=hmv,
where h = Planck’s constant
m= mass of the particle
v = velocity of the particle
(a) It is given that the speed of an electron and proton are equal.
It is clear from the above equation that
λ∝1m As mass of a proton, mp > mass of an electron, me, the proton will have a smaller wavelength compared to the electron.
(b)
λ=hp ∵p=mv So, when the proton and the electron have same momentum, they will have the same wavelength.
(c) de-Broglie wavelength
λis also given by
λ=h2mE,
where E = energy of the particle.
Let the energy of the proton and electron be E.
Wavelength of the proton,
λp=h2mpE …1Wavelength of the electron,
λe=h2meE …2Dividing (2) by (1), we get:
λeλp=memp⇒λeλp<1⇒λe<λpIt is clear that the proton will have smaller wavelength compared to the electron.
Question 13:
If an electron has a wavelength, does it also have a colour?
Answer:
Colour is a characteristic of electromagnetic waves. Electrons behave as a de-Broglie wave because of their velocity. A de-Broglie wave is not an electromagnetic wave and is one dimensional. Hence, no colour is shown by an electron.
Question 1:
Planck’s constant has the same dimensions as
(a) force × time
(b) force × distance
(c) force × speed
(d) force × distance time
Answer:
(d) force × distance time
Planck’s constant,
h =
Ev=Force×distancefrequency
⇒h = force
×distance time
Question 2:
Two photons of
(a) equal wavelength have equal linear momenta
(b) equal energies have equal linear momenta
(c) equal frequencies have equal linear momenta
(d) equal linear momenta have equal wavelengths
Answer:
Two photons having equal linear momenta have equal wavelengths is correct. As in the rest of the options magnitude of momentum or energy can be same because energy and momentum are inversely proportional to wavelength. But the direction of propagation of the photons can be different.
Hence the correct option is D.
Question 3:
Let p and E denote the linear momentum and energy of a photon. If the wavelength is decreased,
(a) both p and E increase
(b) p increases and E decreases
(c) p decreases and E increases
(d) both p and E decrease
Answer:
(a) both p and E increase
From the de-Broglie relation, wavelength,
λ=hp …1
⇒ p=hλHere, h = Planck’s constant
p = momentum of electron
It is clear from the above equation that
p∝1λ.
Thus, if the wavelength
λis decreased, then momentum
pwill be increase.
Relation between momentum and energy:
p=2mEHere, E = energy of electron
m = mass of electron
Substituting the value of p in equation (1), we get:
λ=h2mE⇒E=hλ2m⇒E=h22mλ2Thus, on decreasing
λ, the energy will increase.
Question 4:
Let nr and nb be the number of photons emitted by a red bulb and a blue bulb, respectively, of equal power in a given time.
(a) nr = nb
(b) nr < nb
(c) nr > nb
(d) The information is insufficient to derive a relation between nr and nb.
Answer:
(c) nr > nb
The two bulbs are of equal power. It means that they consume equal amount of energy per unit time.
Now, as the frequency of blue light
fbis higher than the frequency of red light
fr,
hfb>hfr.
Hence, the energy of a photon of blue light is more than the energy of a photon of red light.
Thus, a photon of blue light requires more energy than a photon of red light to be emitted.
For the same energy given to the bulbs in a certain time, the number of photons of blue light will be less than that of red light.
∴nr > nb (As the amount of energy emitted from the two bulb is same)
Question 5:
The equation E = pc is valid
(a) for an electron as well as for a photon
(b) for an electron but not for a photon
(c) for a photon but not for an electron
(d) neither for an electron nor for a photon
Answer:
(c) for a photon but not for an electron
The equation E = pc is valid for a particle with zero rest mass. The rest mass of a photon is zero, but the rest mass of an electron is not zero. So, the equation will be valid for photon, and not electron.
Question 6:
The work function of a metal is hv0. Light of frequency v falls on this metal. Photoelectric effect will take place only if
(a) v ≥ v0
(b) v > 2v0
(c) v < v0
(d) v < v0/2
Answer:
(a) v ≥ v0
As the work function of the metal is hv0, the threshold frequency of the metal is v0.
For photoelectric effect to occur, the frequency of the incident light should be greater than or equal to the threshlod frequency of the metal on which light is incident.
Question 7:
Light of wavelength λ falls on a metal with work-function hc/λ0. Photoelectric effect will take place only if
(a) λ ≥ λ0
(b) λ ≥ 2λ0
(c) λ ≤ λ0
(d) λ < λ0/2
Answer:
(c) λ ≤ λ0
As the work-function of the metal is hc/λ0, its threshold wavelength is λ0.
For photoelectric effect, the wavelength of the incident light should be less than or equal to the threshold wavelength of the metal on which light is incident.
Page No 364:
Question 8:
When stopping potential is applied in an experiment on photoelectric effect, no photoelectric is observed. This means that
(a) the emission of photoelectrons is stopped
(b) the photoelectrons are emitted but are re-absorbed
(c) the photoelectrons are accumulated near the collector plate
(d) the photoelectrons are dispersed from the sides of the apparatus
Answer:
(b) the photoelectrons are emitted but are re-absorbed by the emitter metal
In an experiment on photoelectric effect, the photons incident at the metal plate cause photoelectrons to be emitted. The metal plate is termed as “emitter”. The electrons ejected are collected at the other metal plate called “collector”. When the potential of the collector is made negative with respect to the emitter (or the stopping potential is applied), the electrons emitted from the emitter are repelled by the collector. As a result, some electrons go back to the cathode and the current decreases.
Question 9:
If the frequency of light in a photoelectric experiment is doubled, the stopping potential will
(a) be doubled
(b) be halved
(c) become more than double
(d) become less than double
Answer:
(c) become more than double
According to Einstein’s equation of photoelectric effect,
eV0=hv-φ⇒V0=hv-φe …1Here, V0 = stopping potential
v = frequency of light
φ= work function
Let the new frequency of light be 2ν and the corresponding stopping potential be V0‘.
Therefore,
eV0’=2hv-φV0’=2hv-φe …(2)Multiplying both sides of equation (1) by 2, we get:
2V0=2hv-2φe …(3)Now if we compare (2) and (3), it can be observed that:
2hv-φe>2hv-2φe ⇒V0’>2V0It is clear from the above equation that if the frequency of light in a photoelectric experiment is doubled, the stopping potential will be more than doubled.
Question 10:
The frequency and intensity of a light source are doubled. Consider the following statements.
(A) The saturation photocurrent remains almost the same.
(B) The maximum kinetic energy of the photoelectrons is doubled.
(a) A and B are true.
(b) A is true but B is false.
(c) A is false but B is true.
(d) A and B are false.
Answer:
(b) A is true but B is false.
Saturated current varies directly with the intensity of light. As the intensity of light is increased, a large number of photons fall on the metal surface. As a result, a large number of electrons interact with the photons. As a result, the number of emitted electrons increases and, hence, the current also increases.
At the same time, the frequency of the light source also increases.Also, with the increase in frequency of light, the stopping potential increases as well. This will reduce the current. The combined effect of these two is that the current will remain the same
Hence, A is true.
From the Einstein’s photoelectric equation.
Kmax=hv-φWhere Kmax = kinetic energy of electron
v = frequency of light
φ= work function of metal
It is clear from the above equation. As the frequency of light source is doubled, kinetic energy of electron increases. But, it becomes more than the double.
Hence, B is false.
Question 11:
A point source of light is used in a photoelectric effect. If the source is removed farther from the emitting metal, the stopping potential
(a) will increase
(b) will decrease
(c) will remain constant
(d) will either increase or decrease
Answer:
(c) will remain constant
As the source is removed farther from the emitting metal, the intensity of light will decrease. As the stopping potential does not depend on the intensity of light, it will remain constant.
Question 12:
A point source causes photoelectric effect from a small metal plate. Which of the following curves may represent the saturation photocurrent as a function of the distance between the source and the metal?
Figure
Answer:
From the given curves ,curve (d) is correct.
As the relation between intensity (I) of light and distance (r) is
I∝1r2As the distance between the source and the metal is increased, it will result in decrease in the intensity of light. As the saturation current is directly proportional to the intensity of light (
i∝I), it can be concluded that current varies as
i∝1r2. Thus, curve d is correct.
Question 13:
A non-monochromatic light is used in an experiment on photoelectric effect. The stopping potential
(a) is related to the mean wavelength
(b) is related to the longest wavelength
(c) is related to the shortest wavelength
(d) is not related to the wavelength
Answer:
(c) is related to the shortest wavelength
For photoelectric effect to be observed, wavelength of the incident light
λshould be less than the threshold wavelength
λ0of the metal.
Einstein’s photoelectric equation:
eV0=hcλ0-φHere, V0 = stopping potential
λ0= threshold wavelength
h = Planck’s constant
φ= work-function of metal
It is clear from the above equation that stopping potential is related to the shortest wavelength (threshold wavelength).
Question 14:
A proton and an electron are accelerated by the same potential difference. Let λe and λp denote the de Broglie wavelengths of the electron and the proton, respectively.
(a) λe = λp
(b) λe < λp
(c) λe > λp
(d) The relation between λe and λp depends on the accelerating potential difference.
Answer:
(c) λe > λp
Let me and mp be the masses of electron and proton, respectively.
Let the applied potential difference be V.
Thus, the de-Broglie wavelength of the electron,
λe=h2meeV …1And de-Broglie wavelength of the proton,
λp=h2mpeV …2Dividing equation (2) by equation (1), we get:
λpλe=memp me < mp
∴ λpλe<1
⇒λp<λe
Question 1:
When the intensity of a light source in increased,
(a) the number of photons emitted by the source in unit time increases
(b) the total energy of the photons emitted per unit time increases
(c) more energetic photons are emitted
(d) faster photons are emitted
Answer:
(a) the number of photons emitted by the source in unit time increases
(b) the total energy of the photons emitted per unit time increases
When the intensity of a light source in increased, a large number of photons are emitted from the light source. Hence, option (a) is correct.
Due to increase in the number of photons, total energy of the photons emitted per unit time also increases. Hence, option (b) is also correct.
Increase in the intensity of light increases only the number of photons, not the energy of photons, Hence, option (c) is incorrect.
The speed of photons is not affected by the intensity of light, Hence, option (d) is incorrect.
Question 2:
Photoelectric effect supports quantum nature of light because
(a) there is a minimum frequency below which no photoelectrons are emitted
(b) the maximum kinetic energy of photoelectrons depends only on the frequency of light and not on its intensity
(c) even when the metal surface is faintly illuminated the photoelectrons leave the surface immediately
(d) electric charge of the photoelectrons is quantised
Answer:
(a) there is a minimum frequency below which no photoelectrons are emitted
(b) the maximum kinetic energy of photoelectrons depends only on the frequency of light and not on its intensity
(c) even when the metal surface is faintly illuminated the photoelectrons leave the surface immediately
Photoelectric effect can be explained on the basis of quantum nature of light. According to the quantum nature of light, energy in light is not uniformly spread. It is contained in packets or quanta known as photons.
Energy of a photon, E = hv, where h is Planck’s constant and v is the frequency of light.
Above a particular frequency, called threshold frequency, energy of a photon is sufficient to emit an electron from the metal surface and below which, no photoelectron is emitted, as the energy of the photon is low. Hence, option (a) supports the quantum nature of light.
Now, kinetic energy of an electron,
K=hv0-φThus, kinetic energy of a photoelectron depends only on the frequency of light (or energy). This shows that if the intensity of light is increased, it only increases the number of photons and not the energy of photons. Kinetic energy of photons can be increased by increasing the frequency of light or by increasing the energy of photon, which supports E = hv and, hence, the quantum nature of light. Hence, option (b) also supports the quantum nature of light.
Photoelectrons are emitted from a metal surface even if the metal surface is faintly illuminated; it means that less photons will interact with the electrons. However, few electrons absorb energy from the incident photons and come out from the metal. This shows the quantum nature of light. Hence, (c) also supports the quantum nature of light.
Electric charge of the photoelectrons is quantised; but this statement does not support the quantum nature of light.
Question 3:
A photon of energy hv is absorbed by a free electron of a metal with work-function hv − φ.
(a) The electron is sure to come out.
(b) The electron is sure to come out with kinetic energy hv − φ.
(c) Either the electron does not come out or it comes our with kinetic energy hv − φ.
(d) It may come out with kinetic energy less than hv − φ.
Answer:
(d) It may come out with kinetic energy less than hv − φ.
When light is incident on the metal surface, the photons of light collide with the free electrons. In some cases, a photon can give all the energy to the free electron. If this energy is more then the work-function of the metal,then there are two possibilities. The electron can come out of the metal with kinetic energy hv − φ or it may lose energy on collision with the atoms of the metal and come out with kinetic energy less than hv − φ. Thus, it may come out with kinetic energy less than hv − φ.
Question 4:
If the wavelength of light in an experiment on photoelectric effect is doubled,
(a) photoelectric emission will not take place
(b) photoelectric emission may or may not take place
(c) the stopping potential will increase
(d) the stopping potential will decrease
Answer:
(b) photoelectric emission may or may not take place
(d) the stopping potential will decrease
For photoelectric effect to be observed, wavelength of incident light should not be more than the largest wavelength called threshold wavelength
λ0. If the wavelength of light in an experiment on photoelectric effect is doubled and if it is equal to or less than the threshold wavelength, then photoelectric emission will take place. If it is greater than the threshold wavelength, photoelectric emission will not take place. The photoelectric emission may or may not take place.Photoelectric emission depends on the wavelength of incident light.
Hence, option (b) is correct and (a) is incorrect.
From Einstein’s photoelectric equation,
eV0=hcλ0-φ,
where V0 = stopping potential
λ0= threshold wavelength
h = Planck’s constant
φ= work-function of metal
It is clear that
V0∝1λ0Thus, if the wavelength of light in an experiment on photoelectric effect is doubled, its stopping potential will become half.
Question 5:
The photo current in an experiment on photoelectric effect increases if
(a) the intensity of the source is increased
(b) the exposure time is increased
(c) the intensity of the source is decreased
(d) the exposure time is decreased
Answer:
(a) the intensity of the source is increased
When the intensity of the source is increased, the number of photons emitted from the source increases. As a result, a large number of electrons of the metal interact with these photons and hence, the number of electrons emitted from the metal increases. Thus, the photocurrent in an experiment of photoelectric effect increases. The photocurrent does not depend on the exposure time. Hence, option (a) is correct.
Question 6:
The collector plate in an experiment on photoelectric effect is kept vertically above the emitter plate. A light source is put on and a saturation photocurrent is recorded. An electric field is switched on that has a vertically downward direction.
(a) The photocurrent will increase.
(b) The kinetic energy of the electrons will increase.
(c) The stopping potential will decrease.
(d) The threshold wavelength will increase.
Answer:
(b) The kinetic energy of the electrons will increase.
As there is no effect of electric field on the number of photons emitted, the photoelectric current will remain same. Hence, option (a) is incorrect.
When an electric field is applied, then electric force will act on the electron moving opposite the direction of electric field, which will increase the kinetic energy of the electron. Hence, option (b) is correct.
As the kinetic energy of the electron is increasing, its stopping potential will increase. Hence, option (c) is incorrect.
Threshold wavelength is the characteristic property of the metal and will not change. Hence, (d) is incorrect.
Question 7:
In which of the following situations, the heavier of the two particles has smaller de Broglie wavelength? The two particles
(a) move with the same speed
(b) move with the same linear momentum
(c) move with the same kinetic energy
(d) have fallen through the same height
Answer:
(a) move with the same speed
(c) move with the same kinetic energy
(d) have fallen through the same height
Let m1 be the mass of the heavier particle and m2 be the mass of the lighter particle.
If both the particles are moving with the same speed v, de Broglie wavelength of the heavier particle,
λ1=hm1v …(1)
de Broglie wavelength of the lighter particle,
λ2=hm2v …(2)
Thus, from equations (1) and (2), we find that if the particles are moving with the same speed v, then
λ1<λ2.
Hence, option (a) is correct.
If they are moving with the same linear momentum, then using the de Broglie relation
λ = hpWe find that both the bodies will have the same wavelength. Hence, option (b) is incorrect.
If K is the kinetic energy of both the particles, then de Broglie wavelength of the heavier particle,
λ1=h2m1Kde Broglie wavelength of the lighter particle,
λ2=h2m2KIt is clear from the above equation that if
m1>m2, then
λ1<λ2.
Hence, option (c) is correct.
When they have fallen through the same height h, then velocity of both the bodies,
v =
2ghNow,
λ1=hm12gh
λ2=hm22ghm1>m2
∴ λ1<λ2Hence, option (d) is correct.
Page No 365:
Question 1:
Visible light has wavelengths in the range of 400 nm to 780 nm. Calculate the range of energy of the photons of visible light.
Answer:
Given:
Range of wavelengths,
λ1= 400 nm to
λ2= 780 nm
Planck’s constant, h = 6.63
×10
-34 Js
Speed of light, c = 3
×108 m/s
Energy of photon,
E=hv
ν=cλ
∴ E=hν=hcλ Energy
E1of a photon of wavelength
λ1:
E1=hcλ1 =6.63×10-34×3×108400×10-9 =6.63×34×10-9 =4.97725×10-19 =5×10-19 JEnergy (E2) of a photon of wavelength (
λ2):
E2=6.63×37.8×10-19 =2.55×10-9 JSo, the range of energy is 2.55 × 10−19 J to 5 × 10−19 J.
Question 2:
Calculate the momentum of a photon of light of wavelength 500 nm.
Answer:
Given:
Wavelength of light,
λ= 500 nm
Planck’s constant, h = 6.63
×10
-34J-s
Momentum of a photon of light,
p=hλ =6.63×10-34500×10-9 =6.635×10-27 =1.326×10-27 =1.33×10-27 kg-m/s
Question 3:
An atom absorbs a photon of wavelength 500 nm and emits another photon of wavelength 700 nm. Find the net energy absorbed by the atom in the process.
Answer:
Given:
Wavelength of absorbed photon,
λ1= 500 nm
Wavelength of emitted photon,
λ2= 700 nm
Speed of light, c = 3
×108m/s
Planck’s constant, h = 6.63
×10
-34Js
Energy of absorbed photon,
E1=hcλ1=h×3×108500×10-9Energy of emitted photon,
E2=hcλ2=h×3×108700×10-9Energy absorbed by the atom in the process:
E1-E2=hc1λ1-1λ2 =6.63×315-17×10-19 =6.63×3×235×10-19 =1.136×10-19 J
Question 4:
Calculate the number of photons emitted per second by a 10 W sodium vapour lamp. Assume that 60% of the consumed energy is converted into light. Wavelength of sodium light = 590 nm
Answer:
Given:
Power of the sodium vapour lamp, P = 10 W
Wavelength of sodium light,
λ= 590 nm
Electric energy consumed by the bulb in one second = 10 J
Amount of energy converted into light = 60 %
∴ Energy converted into light =
60100×10=6 JEnergy needed to emit a photon from the sodium atom,
E’=hcλE’=6.63×10-34×3×108590×10-9E’=6.63×3590×10-17 JNumber of photons emitted,
n=66.63×3590×10-17n=6×5906.63×3×1017
n = 1.77 × 1019
Question 5:
When the sun is directly overhead, the surface of the earth receives 1.4 × 103 W m−2 of sunlight. Assume that the light is monochromatic with average wavelength 500 nm and that no light is absorbed in between the sun and the earth’s surface. The distance between the sun and the earth is 1.5 × 1011 m. (a) Calculate the number of photons falling per second on each square metre of earth’s surface directly below the sun. (b) How many photons are there in each cubic metre near the earth’s surface at any instant? (c) How many photons does the sun emit per second?
Answer:
Here,
Intensity of light, I = 1.4 × 103 W/m2,
Wavelength of light,
λ= 500 nm = 500
×10
-9m
Distance between the Sun and Earth, l = 1.5
×1011 m
Intensity,
I=PowerArea=1.4×103 W/m2Let n be the number of photons emitted per second.
∴ Power, P = Energy emitted/second
P=nhcλ,
where
λ= wavelength of light
h = Planck’s constant
c = speed of light
Number of photons/m2 =
nhcλ×A=nhcλ×1= I
∴ n=I×λhc =1.4×103×500×10-96.63×10-34×3×108 =3.5×1021(b) Consider number of two parts at a distance r and r + dr from the source.
Let dt‘ be the time interval in which the photon travels from one part to another.
Total number of photons emitted in this time interval,
N=ndt=Pλhc×AdrcThese points will be between two spherical shells of radius ‘r‘ and r + dr. It will be the distance of the 1st point from the sources.
In this case,
l=1.5×1011 mWavelength, λ=500 nm=500×10-9 mP4πr2=1.4×103∴ No. of photons/m3=P4πr2λhc2=1.4×103×500×10-96.63×10-34×9×1016=1.2×1013(c) Number of photons emitted = (Number of photons / s-m2) × Area
=(3.5×1021)×4πl2=3.5×1021×4×(3.14)×(1.5×1011)2=9.9×1044
Question 6:
A parallel beam of monochromatic light of wavelength 663 nm is incident on a totally reflecting plane mirror. The angle of incidence is 60° and the number of photons striking the mirror per second is 1.0 × 1019. Calculate the force exerted by the light beam on the mirror.
Answer:
Here,
Wavelength of monochromatic light, λ=663×10-9mAngle of incidence, θ=60°Number of photons per second, n=1×1019Momentum of photon,p=hλ,where h is Planck’s constant.p=6.63×10-34663×10-9=10-27Force exerted on the wall,
F=n×pcosθ-(-pcosθ)=2npcosθ =2×1×1019×10-27×12 =1.0×10-8 N
Question 7:
A beam of white light is incident normally on a plane surface absorbing 70% of the light and reflecting the rest. If the incident beam carries 10 W of power, find the force exerted by it on the surface.
Answer:
Power of the incident beam, P = 10 watt
Relation between wavelength
λand momentum (p):
λ=hp,where h is Planck’s constant⇒ p=hλOn dividing both sides by t, we get:pt=hλt …1Energy,
E=hcλ⇒ Et=hcλtLet P be the power. Then,
P=Et=hcλtP=pct [Using equation1]⇒ Pc=ptForce,F=pt=Pc Since F = Momentum Time Force, F=710(absorbed)+2×310(reflected)F=710×Pc+2×310×PcF=710×103×108+2×310×103×108F=133×10-8=4.33×10-8 N
Question 8:
A totally reflecting, small plane mirror placed horizontally faces a parallel beam of light, as shown in the figure. The mass of the mirror is 20 g. Assume that there is no absorption in the lens and that 30% of the light emitted by the source goes through the lens. Find the power of the source needed to support the weight of the mirror.
Figure
Answer:
Given:
Mass of the mirror, m = 20 g = 20 × 10−3 kg
The weight of the mirror will be balanced if the force exerted by the photons will be equal to the weight of the mirror.
Now,
Relation between wavelength
λand momentum (p):
p=hλOn dividing both sides by t, we get:Pt=hλt …1Energy,
E=hcλ⇒ Et=hcλtLet P be the power. Then,
P=Et=hcλtP=pct [Using equation1]⇒ Pc=ptForce,F=Pt=Pc Since F = Momentum TimeThus, rate of change of momentum = Power/c
As the light gets reflected normally,
Force exerted = 2 (Rate of change of momentum) = 2 × Power/c
30%of 2×Powerc=mg⇒ Power=20×10-3×10×3×108×102×3 =100 MW
Question 9:
A 100 W light bulb is placed at the centre of a spherical chamber of radius 20 cm. Assume that 60% of the energy supplied to the bulb is converted into light and that the surface of the chamber is perfectly absorbing. Find the pressure exerted by the light on the surface of the chamber.
Answer:
Given:
Power of the light bulb, P = 100 W
Radius of the spherical chamber, R = 20 cm = 0.2 m
It is given that 60% of the energy supplied to the bulb is converted to light.
Therefore, power of light emitted by the bulb, P’ = 60 W
Force,
F=Pc,
where c is the speed of light
F=603×108=2×10-7 NPressure =
ForceArea
=2×10-74×3.14×(0.2)2 A=4πr2=18×3.14×10-5=0.039×10-5=3.9×10-7=4×10-7 N/m2
Question 10:
A sphere of radius 1.00 cm is placed in the path of a parallel beam of light of large aperture. The intensity of the light is 0.5 W cm−2. If the sphere completely absorbs the radiation falling on it, find the force exerted by the light beam on the sphere.
Answer:
Given:
Radius of the sphere, r = 1 cm
Intensity of light, I = 0.5 Wcm−2
Let A be the effective area of the sphere perpendicular to the light beam.
So, force exerted by the light beam on the sphere is given by,
F=Pc=AIc
F=π×(1)2×0.53×108 =3.14×0.53×108 =0.523×10-8 =5.2×10-9 N
Question 11:
Consider the situation described in the previous problem. Show that the force on the sphere due to the light falling on it is the same even if the sphere is not perfectly absorbing.
Answer:
Consider a sphere of centre O and radius OP. As shown in the figure, the radius OP of the sphere is making an angle θ with OZ. Let us rotate the radius about OZ to get another circle on the sphere. The part of the sphere between the circle is a ring of area
2πr2sinθdθ.
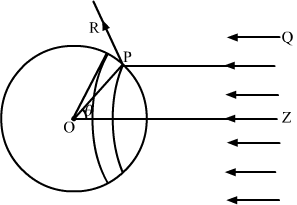
Consider a small part of area
∆Aof the ring at point P.
Energy of the light falling on this part in time
∆t,
∆U=I∆t∆AcosθAs the light is reflected by the sphere along PR, the change in momentum,
∆p=2∆Uccosθ=2cI∆t∆Acos2θTherefore, the force will be
∆p∆t=2cI∆Acos2θThe component of force on
∆A, along ZO, is
∆p∆tcosθ=2cI∆Acos3θNow, force action on the ring,
dF=2cI2πr2sinθdθcos3θThe force on the entire sphere,
F=∫0π24πr2Iccos3θsinθdθ =-∫0π24πr2Iccos3θdcosθ=πr2IcThis is same as given in the previous problem.
Question 12:
Show that it is not possible for a photon to be completely absorbed by a free electron.
Answer:
When an electron undergoes an inelastic collision with a photon, we can apply the principle of conservation of energy to this collision. So,
pc+mec2=p2c2+me2c4 …(i)
Here, h = Planck’s constant
c = the speed of light
me = rest mass of electron
pc = energy of the photon
Squaring on both side of equation (i),
(pc+mec2)2=p2c2+me2c4⇒p2c2+me2c4+2(pc)(mec2)=p2c2+me2c4⇒2(pc)(mec2)=0 or pc=0 ( as ‘m’ and ‘c’ are non zero)This gives vanishing energy of photon which is not possible.
Question 13:
Two neutral particles are kept 1 m apart. Suppose by some mechanism some charge is transferred from one particle to the other and the electric potential energy lost is completely converted into a photon. Calculate the longest and the next smaller wavelength of the photon possible.
Answer:
Given:
Distance between the two neutral particles, r = 1 m
Electric potential energy,
E1=
kq2r=
kq2,
where k =
14πε0Energy of photon,
E2 =
hcλ,
where
λ= wavelength of light
h = Planck’s constant
c = speed of light
Here, E1 = E2
∴ kq2=hcλ⇒ λ=hckq2For wavelength, λ, to be maximum, charge q should be minimum.
q = e=1.6×10-19 C Maximum wavelength, λ=hckq2 =6.63×3×10-34×1089×102×(1.6)2×10-38 =0.863×103=863 m Next smaller wave length,
λ=6.63×3×10-34×1089×104×4×(1.6)2×10-38 =8634 =215.74 m
Question 14:
Find the maximum kinetic energy of the photoelectrons ejected when light of wavelength 350 nm is incident on a cesium surface. Work function of cesium = 1.9 eV
Answer:
Given:
Wavelength of light, λ = 350 nm = 350 × 10−9 m
Work-function of cesium, Ï• = 1.9 eV
From Einstein’s photoelectric equation,
E=ϕ+Kinetic energy of electron⇒K E=E-ϕ⇒K E=hcλ-ϕ,where λ=wavelength of light h= Planck’s constantMaximum kinetic energy of electrons,
Emax=hcλ-ϕEmax=6.63×10-34×3×108350×10-9×1.6×10-19-1.9Emax=6.63×3×102350×1.6-1.9Emax=1.65 eV=1.6 eV
Question 15:
The work function of a metal is 2.5 × 10−19 J. (a) Find the threshold frequency for photoelectric emission. (b) If the metal is exposed to a light beam of frequency 6.0 × 1014 Hz, what will be the stopping potential?
Answer:
Given:
Work function of a metal, W0 = 2.5 × 10−19 J
Frequency of light beam, v = 6.0 × 1014 Hz
(a) Work function of a metal,
W0 = hv0,
where h = Planck’s constant
v0 = threshold frequency
∴v0 =
W0h
⇒v0=2.5×10-196.63×10-34 =3.77×1014 Hz =3.8×1014 Hz(b) Einstein’s photoelectric equation:
eV0=hv-W0,
where v = frequency of light
V0 = Stopping potential
e = charge on electron
∴ V0=hv-W0e =6.63×10-34×6×1014-2.5×10-191.6×10-19 =3.97×10-19-2.5×10-191.6×10-19=0.91 V
Question 16:
The work function of a photoelectric material is 4.0 eV. (a) What is the threshold wavelength? (b) Find the wavelength of light for which the stopping potential is 2.5 V.
Answer:
Work function of a photoelectric material, Ï• = 4 eV = 4 × 1.6 × 10−19 J
Stopping potential, V0 = 2.5 V
Planck’s constant, h = 6.63
×10-34 Js(a) Work function of a photoelectric material,
ϕ=hcλ0 Here, λ0 = threshold wavelength of light
c = speed of light
∴λ0=hcϕλ0=6.63×10-34×3×1084×1.6×10-19λ0=6.63×364×10-2710-9λ0= 3.1×10-7mλ0= 310 nm(b) From Einstein’s photoelectric equation,
E=ϕ+eV0On substituting the respective values, we get: hcλ=4×1.6×10-19+1.6×10-19×2.5⇒λ=6.63×10-34×3×1086.5×1.6×10-19⇒λ=6.63×3×10-261.6×10-19×6.5⇒λ=1.9125×10-7=191 nm
Question 17:
Find the maximum magnitude of the linear momentum of a photoelectron emitted when a wavelength of 400 nm falls on a metal with work function 2.5 eV.
Answer:
Given:
Wavelength of light,
λ= 400 nm = 400
×10
-9m
Work function of metal,
ϕ= 2.5 eV
From Einstein’s photoelectric equation,
Kinetic energy=hcλ-ϕHere, c = speed of light
h = Planck’s constant
∴K.E.=6.63×10-34×3×1084×10-7×1.6×10-19-2.5 eV =0.605 eV Also, K.E. =
p22m,
where p is momentum and m is the mass of an electron.
∴ p2=2m×K.E.⇒p2=2×9.1×10-31×0.605×1.6×10-19⇒p=4.197×10-25 kg-m/s
Question 18:
When a metal plate is exposed to a monochromatic beam of light of wavelength 400 nm, a negative potential of 1.1 V is needed to stop the photo current. Find the threshold wavelength for the metal.
Answer:
Given:
Wavelength of light,
λ= 400 nm = 400
×10
-9m
Stopping potential, V0 = 1.1 V
From Einstein’s photoelectric equation,
hcλ=hcλ0+eV0,
where h = Planck’s constant
c= speed of light
λ= wavelength of light
λ0= threshold wavelength
V0= stopping potential
On substituting the respective values in the above formula, we get:
6.63×10-34×3×108400×10-9=6.63×10-34×3×108λ0+1.6×10-19×1.1⇒ 4.97×10-19=19.89×10-26λ0+1.76×10-19⇒ 4.97=19.89×10-7λ0+1.76⇒ 19.89×10-7λ0=4.97-1.76=3.21⇒ λ0=19.89×10-73.21 =6.196×10-7 m=620 nm
Question 19:
In an experiment on photoelectric effect, the stopping potential is measured for monochromatic light beams corresponding to different wavelengths. The data collected are as follows:
Wavelength (nm): 350 400 450 500 550
Stopping potential (V): 1.45 1.00 0.66 0.38 0.16
Plot the stopping potential against inverse of wavelength (1/λ) on a graph paper and find (a) Planck’s constant (b) the work function of the emitter and (c) the threshold wavelength.
Answer:
(a)
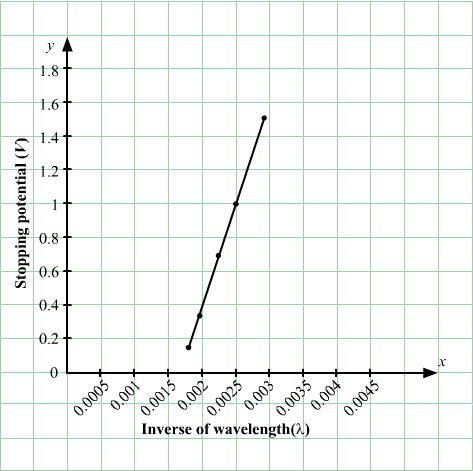
When λ = 350, Vs = 1.45
and when λ = 400, Vs = 1
∴ hc350=w+1.45 …(1)and hc400=w+1 …(2)Subtracting (2) from (1) and solving to get the value of h, we get:
h = 4.2 × 10−15 eV-s
(b) Now, work function,
w=12240350-1.45=2.15ev(c) w=ncλ⇒λthreshold=hcw⇒λthreshold=12401.15⇒λthreshold=576.8 nm
Question 20:
The electric field associated with a monochromatic beam is 1.2 × 1015 times per second. Find the maximum kinetic energy of the photoelectrons when this light falls on a metal surface whose work function is 2.0 eV.
Answer:
Given:
Electric field of the monochromatic beam, E = 1.2 × 1015 times per second
Frequency, v =
1.2×10152=0.6×1015 HzWork function of the metal surface,
ϕ= 2.0 eV
From Einstein’s photoelectric equation, kinetic energy,
K=hv-ϕ0⇒ K=6.63×10-34×0.6×1015 1.6×10-19-2 =0.486 eVThus, the maximum kinetic energy of a photon is 0.486 eV.
Question 21:
The electric field associated with a light wave is given by
E=E0 sin [(1.57×107m-1)(x-ct)].Find the stopping potential when this light is used in an experiment on photoelectric effect with the emitter having work function 1.9 eV.
Answer:
Given:
Electric field, E=E0 sin [(1.57×107m-1)(x-ct)]Work function,
ϕ= 1.9 eV
On comparing the given equation with the standard equation,
E=E0sinkx-wt, we get:
ω=1.57×107×c
Now, frequency,
v=1.57×107×3×1082π HzFrom Einstein’s photoelectric equation,
eV0=hv-ϕHere, V0 = stopping potential
e = charge on electron
h = Planck’s constant
On substituting the respective values, we get:
eV0=6.63×10-34×1.57×3×10152π×1.6×10-19-1.9 eV⇒eV0=3.105-1.9=1.205 eV⇒V0=1.205×1.6×10-191.6×10-19=1.205 VThus, the value of the stopping potential is 1.205 V.
Page No 366:
Question 22:
The electric field at a point associated with a light wave is
E=(100Vm-1) sin [(3.0×1015 s-1)t] sin [(6.0 ×1015 s-1)t].If this light falls on a metal surface with a work function of 2.0 eV, what will be the maximum kinetic energy of the photoelectrons?
Answer:
Given:
E=100sin [(3×10-15s-1)t] sin [(6×10-15s-1)t] =100×12cos[(9×1015s-1)t]-cos[(3×1015s-1)t]
The values of angular frequency
ωare 9 × 1015 and 3 × 1015 .
Work function of the metal surface,
ϕ= 2 eV
Maximum frequency,
v=ωmax2π=
9×10152πHz
From Einstein’s photoelectric equation, kinetic energy,
K = hv
– ϕ
⇒K =
6.63×10-34×9×10152π×11.6×10-19 -2 eV
⇒K = 3.938 eV
Question 23:
A monochromatic light source of intensity 5 mW emits 8 × 1015 photons per second. This light ejects photoelectrons from a metal surface. The stopping potential for this setup is 2.0 V. Calculate the work function of the metal.
Answer:
Given:
Intensity of light, I = 5 mW
Number of photons emitted per second, n = 8 × 1015
Stopping potential, V0 = 2 V
Energy, E =
hv=
In = 5×10-38×1015From Einstein’s photoelectric equation, work function,
W0=hv-eV0Here, h = Planck’s constant
e = 1.6
×10
-19C
On substituting the respective values, we get:
W0=5×10-38×1015-1.6×10-19×2 =6.25×10-19-3.2×10-19 =3.05×10-19 =3.05×10-191.6×10-15=1.906 eV
Question 24:
The figure is the plot of stopping potential versus the frequency of the light used in an experiment on photoelectric effect. Find (a) the ratio h/e and (b) the work function.
Figure
Answer:
We have to take two cases.
CaseI When stopping potential, V0=1.656 voltsFrequency, v=5×1014 HzCaseII When stopping potential, V0=0Frequency, v=1×1014 Hz(b)From Einstein’s equation, eV0=hv-W0On substituting the values of case1 and case2, we get:1.656e=h×5×1014-W0 …1 0=5×h×1×1014-5×W0 …2Subtracting equation2 from 1, we get: W0=1.6564 eV =0.414 eV(a) Putting the value of W0 in equation (2), we get:
⇒ 5W0=5h×1014⇒ 5×0.414=5×h×1014⇒ h=4.414×10-15 eVsOr he=4.414×10-15 Vs
Question 25:
A photographic film is coated with a silver bromide layer. When light falls on this film, silver bromide molecules dissociate and the film records the light there. A minimum of 0.6 eV is needed to dissociate a silver bromide molecule. Find the maximum wavelength of light that can be recorded by the film.
Answer:
Given:
Work function, W0 = 0.6 eV
Now, work function,
W0=hcλ,
where, h = Planck’s constant
λ= wavelength of light
c = speed of light
∴λ=hcW0 =6.63×10-34×3×1080.6×1.6×10-19 =20.71×10-7 m =2071 nm
Question 26:
In an experiment on photoelectric effect, light of wavelength 400 nm is incident on a cesium plate at the rate of 5.0 W. The potential of the collector plate is made sufficiently positive with respect to the emitter, so that the current reaches its saturation value. Assuming that on average, one out of every 106 photons is able to eject a photoelectron, find the photocurrent in the circuit.
Answer:
Given:
Wavelength of light, λ = 400 nm
Power, P = 5 W
Energy of photon,
E =
hcλ=1242400 eVNumber of photons, n =
PE
n=5×4001.6×10-19×1242Number of electrons = 1 electron per 106 photons
Number of photoelectrons emitted,
n’=5×4001.6×1242×10-19×106Photo electric current,
I = Number of electron
×Charge on electron
I=5×4001.6×1242×10-19×106×1.6×10-19 =1.6×10-6 A=1.6 μA
Question 27:
A silver ball of radius 4.8 cm is suspended by a thread in a vacuum chamber. Ultraviolet light of wavelength 200 nm is incident on the ball for some time during which light energy of 1.0 × 10−7 J falls on the surface. Assuming that on average, one photon out of every ten thousand is able to eject a photoelectron, find the electric potential at the surface of the ball, assuming zero potential at infinity. What is the potential at the centre of the ball?
Answer:
Given:
Radius of the silver ball, r = 4.8 cm
Wavelength of the ultra violet light, λ = 200 nm = 2 × 10−7 m
Total energy of light, E = 1.0 × 10−7 J
We are given that one photon out of every ten thousand is able to eject a photoelectron.
Energy of one photon,
E’=hcλ,
where h = Planck’s constant
c = speed of light
λ= wavelength of light
On substituting the respective values in the above formula, we get:
E’=6.63×10-34×3×1082×10-7 =9.945×10-19
Number of photons,
n=EE’=1×10-79.945×10-19=1×1011
Number of photoelectrons
=1×1011104=1×107The amount of positive charge developed due to the outgoing electrons,
q=1×107×1.6×10-19 =1.6×10-12 CPotential developed at the centre as well as on surface,
V=Kqr,
where K =
14πε0
∴ V=9×109×1.6×10-124.8×10-2 =0.3 VPotential inside the silver ball remains constant. Therefore, potential at the centre of the sphere is 0.3 V.
Question 28:
In an experiment on photoelectric effect, the emitter and the collector plates are placed at a separation of 10 cm and are connected through an ammeter without any cell. A magnetic field B exists parallel to the plates. The work function of the emitter is 2.39 eV and the light incident on it has wavelengths between 400 nm and 600 nm. Find the minimum value of B for which the current registered by the ammeter is zero. Neglect any effect of space charge.
Figure
Answer:
Given:
Separation between the collector and emitter, d = 10 cm
Work function, Ï• = 2.39 eV
Wavelength range, λ1 = 400 nm to λ2 = 600 nm
Magnetic field B will be minimum if energy is maximum.
For maximum energy, wavelength λ should be minimum.
Einstein’s photoelectric equation:
E=hcλ-ϕHere, h=Planck’s constant λ=Wavelength of light c=speed of light∴ E=1242400-2.39 =3.105-2.39=0.715 eVThe beam of ejected electrons will be bent by the magnetic field. If the electrons do not reach the other plates, there will be no current.
When a charged particle is sent perpendicular to a magnetic field, it moves along a circle of radius,
r=mvqB,
where m = mass of charge particle
B = magnetic field
v = velocity of particle
q = charge on the particle
Radius of the circle should be equal to r = d, so that no current flows in the circuit.
⇒ r=2mEqB ∵mv=2mE ⇒0.1=2×9.1×10-31×1.6×10-19×0.7151.6×10-19×B⇒B=2×9.1×1.6×0.715×10-501.6×10-20⇒B=2×9.1×1.6×0.7151.6×10-5⇒B=2.85×10-5 T
Question 29:
In the arrangement shown in the figure, y = 1.0 mm, d = 0.24 mm and D = 1.2 m. The work function of the material of the emitter is 2.2 eV. Find the stopping potential V needed to stop the photocurrent.
Figrue]
Answer:
Given:
Fringe width, y = 1 mm
×2 = 2 mm
Work function, W0 = 2.2 eV
D = 1.2 m
d = 0.24 mm
Fringe width,
y=λDd ,
where
λ= wavelength of light
∴ λ=2×10-3×0.24×10-31.2 =4×10-7 m
Energy, E=hcλ =4.14×10-15×3×1084×10-7 =3.105 eVFrom Einstein’s photoelectric equation,
eV0=E-W0,
where V0 is the stopping potential and e is charge of electron.
∴ eV0=3.105-2.2=0.905 eV V0=0.9051.6×10-19×1.6×10-19 V =0.905 V
Question 30:
In a photoelectric experiment, the collector plate is at 2.0 V with respect to the emitter plate made of copper (φ = 4.5 eV). The emitter is illuminated by a source of monochromatic light of wavelength 200 nm. Find the minimum and maximum kinetic energy of the photoelectrons reaching the collector.
Answer:
Given:
Work function of copper, Ï• = 4.5 eV,
Wavelength of monochromatic light, λ = 200 nm
From Einstein’s photoelectric equation, kinetic energy,
K=E-ϕ=hcλ-ϕ,where, h=Planck’s constant c=speed of light∴ K=1242200-4.5 =6.21-4.5=1.71 eVThus, at least 1.7 eV is required to stop the electron. Therefore, minimum kinetic energy will be 2 eV.
It is given that electric potential of 2 V is required to accelerate the electron. Therefore, maximum kinetic energy
=(2+1.7) eV=3.7 eV
Question 31:
A small piece of cesium metal (φ = 1.9 eV) is kept at a distance of 20 cm from a large metal plate with a charge density of 1.0 × 10−9 C m−2 on the surface facing the cesium piece. A monochromatic light of wavelength 400 nm is incident on the cesium piece. Find the minimum and maximum kinetic energy of the photoelectrons reaching the large metal plate. Neglect any change in electric field due to the small piece of cesium present.
Answer:
Given:
Charge density of the metal plate,
σ= 1.0 × 10−9 Cm−2
Work function of the cesium metal, φ = 1.9 eV
Wavelength of monochromatic light,
λ= 400 nm = 400
×10
-9 m
Distance between the metal plates, d = 20 cm = 0.20 m
Electric potential due to a charged plate,
V = E × d,
where E, the electric field due to the charged plate, is
σε0and
d is the separation between the plates.
∴ V=σε0×d =1×10-9×208.85×10-12×100 ∵ε0=8.65×10-12 C2 N-1-m-2 =22.598 V=22.6 VFrom Einstein’s photoelectric equation,eVo=hv-W0 =hcλ-WOn substituting the respective values, we get:V0 =4.14×10-15×3×1084×10-7-1.9 =3.105-1.9=1.205 eV =1.205 VAs V0 is much less than ‘V‘, the minimum energy required to reach the charged plate must be equal to 22.7eV.
For maximum KE, ‘V’ must have an accelerating value.
Hence maximum kinetic energy,
K.E.=V0+V=1.205+22.6 =23.8005 eV
Question 32:
Consider the situation of the previous problem. Consider the faster electron emitted parallel to the large metal plate. Find the displacement of this electron parallel to its initial velocity before it strikes the large metal plate.
Answer:
Electric field of the metal plate,
E=σε0=1×10-98.85×10-12 =113 V/mAcceleration, a=qEm,where q=charge on electron E=electric field m=mass of electrona=1.6×10-19×1139.1×10-31=19.87×1012t=2ya=2×20×10-219.87×1012=1.41×10-7 sFrom Einstein’s photoelectric equation, K.E.=hcλ-W=1.2 eV=1.2×1.6×10-19 J[∵ in problem 31:KE=1.2eV]∴Velocity, v=2KEm =2×1.2×1.6×10-194.1×10-312×1.2×1.6×10-194.1×10-31=0.665×10-6 m/s∴ Horizontal displacement,
S=v×tS=0.665×10-6×1.4×10-7S=0.092 m=9.2 cm
Question 33:
A horizontal cesium plate (φ = 1.9 eV) is moved vertically downward at a constant speed v in a room full of radiation of wavelength 250 nm and above. What should be the minimum value of v so that the vertically-upward component of velocity is non-positive for each photoelectron?
Answer:
Given:
Work function of the cesium plate, φ = 1.9 eV
Wavelength of radiation, λ = 250 nm
Energy of a photon,
E=hcλ,where h=Planck’s constant c=speed of light∴E=1240250=4.96 eVFrom Einstein’s photoelectric equation, kinetic energy of an electron,
K=E-ϕ⇒ K=hcλ-ϕ Here, h is Planck’s constant and c is the speed of light⇒K=4.96 eV-1.9 eV =3.06 eV.For non-positive velocity of each photo electron, the velocity of a photoelectron should be equal to minimum velocity of the plate.
∴ Velocity of the photoelectron,
v=2Km m=mass of electron∴ v=2×3.06×1.6×10-199.1×10-31 =1.04×106 ms-1
Question 34:
A small metal plate (work function φ) is kept at a distance d from a singly-ionised, fixed ion. A monochromatic light beam is incident on the metal plate and photoelectrons are emitted. Find the maximum wavelength of the light beam, so that some of the photoelectrons may go round the ion along a circle.
Answer:
From Einstein’s photoelectric equation,
eV0=hcλ-ϕ⇒V0=hcλ-ϕ1eHere, V0 = stopping potential
h = Planck’s constant
c = speed of light
ϕ= work function
The particle will move in a circle when the stopping potential is equal to the potential due to the singly charged ion at that point so that the particle gets the required centripetal force for its circular motion.
⇒Ke2d=hcλ-ϕ1e⇒ Ke22d=hcλ-ϕ⇒hcλ=Ke22d+ϕ=Ke2+2dϕ2d⇒λ=(hc)(2d)ke2+2dϕ⇒λ=2hdc14π∈0e2+2dϕ⇒λ=8π∈0hcde2+8π∈0dϕ
Question 35:
A light beam of wavelength 400 nm is incident on a metal plate of work function 2.2 eV. (a) A particular electron absorbs a photon and makes two collisions before coming out of the metal. Assuming that 10% of the extra energy is lost to the metal in each collision, find the kinetic energy of this electron as it comes out of the metal. (b) Under the same assumptions, find the maximum number of collisions the electron can suffer before it becomes unable to come out of the metal.
Answer:
Given:
Wavelength of light beam,
λ= 400 nm
Work function of metal plate,
ϕ= 2.2 eV
Energy of the photon,
E=hcλ,where h=Planck’s constant c=speed of light∴E=1240400=3.1 eVThis energy will be supplied to the electrons.
Energy lost by the electron in the first collision
= 3.1 eV × 10%
= 0.31 eV
Now, the energy of the electron after the first collision = 3.1
-0.31 = 2.79 eV
Energy lost by electron in the second collision
= 2.79 eV× 10%
= 0.279 eV
Total energy lost by the electron in two collisions
= 0.31 + 0.279 = 0.589 eV
Using Einstein’s photoelectric equation, kinetic energy of the photoelectron when it comes out from the metal,
K=E-ϕ-energy lost due to collisions= (3.1 − 2.2 − 0.589) eV
= 0.31 eV
(b) Similarly for the third collision, the energy lost = (2.79
-0.279) eV × 10%
= 0.2511 eV
Energy of the electron after the third collision = 2.790
-0.2511 = 2.5389
Energy lost in the fourth collision = 2.5389 × 10%
Energy of the electron after the fourth collision = 2.5389
-0.25389 = 2.28501
This value is very close to the work function of the metal plate. After the fifth collision, the energy of the electron becomes less than the work function of the metal.
Therefore, the electron can suffer maximum four collisions before it becomes unable to come out of the metal.
Chapterwise HC Verma Solutions Class 12 Physics :
- Chapter 23 – Heat and Temperature
- Chapter 24 – Kinetic Theory of Gases
- Chapter 25 – Calorimetry
- Chapter 26 – Laws of Thermodynamics
- Chapter 27 – Specific Heat Capacities of Gases
- Chapter 28 – Heat Transfer
- Chapter 29 – Electric Field and Potential
- Chapter 30 – Gauss’s Law
- Chapter 31 – Capacitors
- Chapter 32 – Electric Current in Conductors
- Chapter 33 – Thermal and Chemical Effects of Electric Current
- Chapter 34 – Magnetic Field
- Chapter 35 – Magnetic Field due to a Current
- Chapter 36 – Permanent Magnets
- Chapter 37 – Magnetic Properties of Matter
- Chapter 38 – Electromagnetic Induction
- Chapter 39 – Alternating Current
- Chapter 40 – Electromagnetic Waves
- Chapter 41 – Electric Current through Gases
- Chapter 42 – Photoelectric Effect and Wave Particle Duality
- Chapter 43 – Bohr’s Model and Physics of the Atom
- Chapter 44 – X-rays
- Chapter 45 – Semiconductors and Semiconductor Devices
- Chapter 46 – The Nucleus
- Chapter 47 – The Special Theory of Relativity
About the Author – HC Verma
HC Verma, the author of many popular and well-renowned Physics books, was born on 8 April 1952. Passing out from one of the most prestigious colleges of the country, IIT Kanpur, he worked as an experimental physicist in the Department of Nuclear Physics.
His most famous works which he is known for include the two-volume Concepts of Physics. He also worked for the social upliftment of the economically weaker children through his organization named Shiksha Sopan. He is also the recipient of the Padma Shri, which is considered India’s fourth-highest civilian award. He received the same because of his contribution and valuable work in the field of Physics.
