HC Verma Physics books are the most preferred books among students of CBSE schools. Students can be found referring to the chapters as well as practice questions at the end of each of these chapters, in the books. Students follow these textbooks religiously since quite a few questions in these also appear in exams.
Contents
- 1 HC Verma Solutions for Class 12 Physics Chapter 27 – Specific Heat Capacities of Gases
- 1.0.1 Page No 76:
- 1.0.2 Question 1:
- 1.0.3 Answer:
- 1.0.4 Question 2:
- 1.0.5 Answer:
- 1.0.6 Question 3:
- 1.0.7 Answer:
- 1.0.8 Question 4:
- 1.0.9 Answer:
- 1.0.10 Question 5:
- 1.0.11 Answer:
- 1.0.12 Question 6:
- 1.0.13 Answer:
- 1.0.14 Question 7:
- 1.0.15 Answer:
- 1.0.16 Question 8:
- 1.0.17 Answer:
- 1.0.18 Question 9:
- 1.0.19 Answer:
- 1.0.20 Question 10:
- 1.0.21 Answer:
- 1.0.22 Question 1:
- 1.0.23 Answer:
- 1.0.24 Question 2:
- 1.0.25 Answer:
- 1.0.26 Question 3:
- 1.0.27 Answer:
- 1.0.28 Question 4:
- 1.0.29 Answer:
- 1.0.30 Question 5:
- 1.0.31 Answer:
- 1.0.32 Question 6:
- 1.0.33 Answer:
- 1.0.34 Question 7:
- 1.0.35 Answer:
- 1.0.36 Question 8:
- 1.0.37 Answer:
- 1.0.38 Question 9:
- 1.0.39 Answer:
- 1.0.40 Question 10:
- 1.0.41 Answer:
- 1.0.42 Question 11:
- 1.0.43 Answer:
- 1.0.44 Question 12:
- 1.0.45 Answer:
- 1.0.46 Page No 77:
- 1.0.47 Question 13:
- 1.0.48 Answer:
- 1.0.49 Question 1:
- 1.0.50 Answer:
- 1.0.51 Question 2:
- 1.0.52 Answer:
- 1.0.53 Question 3:
- 1.0.54 Answer:
- 1.0.55 Question 4:
- 1.0.56 Answer:
- 1.0.57 Question 5:
- 1.0.58 Answer:
- 1.0.59 Question 6:
- 1.0.60 Answer:
- 1.0.61 Question 7:
- 1.0.62 Answer:
- 1.0.63 Question 1:
- 1.0.64 Answer:
- 1.0.65 Question 2:
- 1.0.66 Answer:
- 1.0.67 Question 3:
- 1.0.68 Answer:
- 1.0.69 Question 4:
- 1.0.70 Answer:
- 1.0.71 Question 5:
- 1.0.72 Answer:
- 1.0.73 Page No 78:
- 1.0.74 Question 6:
- 1.0.75 Answer:
- 1.0.76 Question 7:
- 1.0.77 Answer:
- 1.0.78 Question 8:
- 1.0.79 Answer:
- 1.0.80 Question 9:
- 1.0.81 Answer:
- 1.0.82 Question 10:
- 1.0.83 Answer:
- 1.0.84 Question 11:
- 1.0.85 Answer:
- 1.0.86 Question 12:
- 1.0.87 Answer:
- 1.0.88 Question 13:
- 1.0.89 Answer:
- 1.0.90 Question 14:
- 1.0.91 Answer:
- 1.0.92 Question 15:
- 1.0.93 Answer:
- 1.0.94 Question 16:
- 1.0.95 Answer:
- 1.0.96 Question 17:
- 1.0.97 Answer:
- 1.0.98 Question 18:
- 1.0.99 Answer:
- 1.0.100 Question 19:
- 1.0.101 Answer:
- 1.0.102 Question 20:
- 1.0.103 Answer:
- 1.0.104 Question 21:
- 1.0.105 Answer:
- 1.0.106 Question 22:
- 1.0.107 Answer:
- 1.0.108 Page No 79:
- 1.0.109 Question 23:
- 1.0.110 Answer:
- 1.0.111 Question 24:
- 1.0.112 Answer:
- 1.0.113 Question 25:
- 1.0.114 Answer:
- 1.0.115 Question 26:
- 1.0.116 Answer:
- 1.0.117 Question 27:
- 1.0.118 Answer:
- 1.0.119 Question 28:
- 1.0.120 Answer:
- 1.0.121 Question 29:
- 1.0.122 Answer:
- 1.0.123 Question 30:
- 1.0.124 Answer:
- 1.0.125 Question 31:
- 1.0.126 Answer:
- 1.0.127 Page No 80:
- 1.0.128 Question 32:
- 1.0.129 Answer:
- 1.0.130 Question 33:
- 1.0.131 Answer:
- 1.0.132 Question 34:
- 1.0.133 Answer:
- 1.0.134 Question 35:
- 1.0.135 Answer:
- 2 Chapterwise HC Verma Solutions Class 12 Physics :
- 3 About the Author – HC Verma
HC Verma Solutions for Class 12 Physics Chapter 27 – Specific Heat Capacities of Gases
For such popular books, students can get extremely helpful practice material online. For all the questions in the HC Verma books, there are several sources where students can get detailed solutions and solve their doubts and queries.
Please note that these solutions are provided here for free.
Page No 76:
Question 1:
Does a gas have just two specific heat capacities or more than two? Is the number of specific heat capacities of a gas countable?
Answer:
No, a gas doesn’t have just two specific heat capacities, as the heat capacities depend on the process followed. There are infinite processes; therefore, there can be infinite number of specific heat capacities.
Question 2:
Can we define specific heat capacity at constant temperature?
Answer:
Specific heat capacity,
s=ΔQm ΔT, where
ΔQ/m is the heat supplied per unit mass of the substance and
ΔT is the change in temperature produced. At constant temperature, ​
ΔT = 0; therefore, s = infinity. So, we cannot define specific heat capacity at constant temperature.
Question 3:
Can we define specific heat capacity for an adiabatic process?
Answer:
Specific heat capacity,
s=ΔQm ΔT, where
∆Q/m is the heat supplied per unit mass of the substance and
∆T is the change in temperature produced. In an adiabatic process, no heat exchange is allowed; so,
∆Q = 0 and hence, s = 0. Therefore, in an adiabatic process, specific heat capacity is zero.
Question 4:
Does a solid also have two kinds of molar heat capacities Cp and Cv? If yes, is Cp > Cv? Or is Cp − Cv = R?
Answer:
Yes, a solid also has two kinds of molar heat capacities, Cpand Cv. In a solid, expansion coefficient is quite small; therefore dependence of heat capacity on the process is negligible. So, Cp> Cvwith just a small difference, which is not equal to R.
Question 5:
In a real gas, the internal energy depends on temperature and also on volume. The energy increases when the gas expands isothermally. Examining the derivation of Cp − Cv = R, find whether Cp − Cv will be more than R, less than R or equal to R for a real gas.
Answer:
In a real gas, as the internal energy depends on temperature and volume, the derived equation for an ideal gas
(dQ)p=(dQ)v+nR dT will change to ​
(dQ)p=(dQ)v+nR dT + k, where k is the change in internal energy (positive) due to change in volume when pressure is kept constant. So, in the case of a real gas, for n=1 mole (say),
Cp-Cv=R+k dT⇒Cp-Cv>R,where Cpand Cvare the specific heat capacities at constant pressure and volume, respectively.
Question 6:
Can a process on an ideal gas be both adiabatic and isothermal?
Answer:
According to the first law of thermodynamics, change in internal energy,
∆U is equal to the difference between heat supplied to the gas,
∆Q and the work done on the gas,​
∆W, such that
ΔQ=ΔU+ΔW​. In an adiabatic process,
∆Q = 0 and in an isothermal process, change in temperature,
∆T = 0. Therefore,
ΔQ=ΔU+ΔW⇒ΔQ=nCvΔT+ΔW⇒0=nCv(0)+ΔW⇒ΔW=0,where Cv is the heat capacity at constant volume.
This shows that if the process is adiabatic as well as isothermal, no work will be done. So, a process on an ideal gas cannot be both adiabatic and isothermal.
Question 7:
Show that the slope of the p−V diagram is greater for an adiabatic process compared to an isothermal process.
Answer:
In an isothermal process,
PV = k …(i)
On differentiating it w.r.t V, we get
V dPdV+P=0 dPdV=-PV dPdV=-kV2 [Using (i)], k = constantk = constant
In an adiabatic process,
PVγ=K …. (ii)On differentiating it w.r.t V, we get
Vγ dPdV+γPVγ-1=0 dPdV=- γPVγ-1Vγ dPdV=-γKVγ+1 [Using (ii)], γ>1 andK is constant
γ and dPdV are the slope of the curve and the ratio of heat capacities at constant pressure and volume, respectively; P is pressure and V is volume of the system.
By comparing the two slopes and keeping in mind that
γ>1, we can see that the slope of the P-V diagram is greater for an adiabatic process than an isothermal process.
Question 8:
Is a slow process always isothermal? Is a quick process always adiabatic?
Answer:
For an isothermal process, PV = K, where P is pressure, V is volume of the system and K is constant. In an isothermal process, a small change in V produces only a small change in p, so as to keep the product constant. On the other hand, in an adiabatic process,
PVγ=k, γ=CPCV > 1 is the ratio of heat capacities at constant pressure and volume, respectively, and k is a constant. In this process, a small increase in volume produces a large decrease in pressure. Therefore, an isothermal process is considered to be a slow process and an adiabatic process a quick process.
Question 9:
Can two states of an ideal gas be connected by an isothermal process as well as an adiabatic process?
Answer:
For two states to be connected by an isothermal process,
P1V1=P2V2 …(i)
For the same two states to be connected by an adiabatic process,
P1V1γ = P2V2γ …(ii)
If both the equations hold simultaneously then, on dividing eqaution (ii) by (i) we get
V1γ-1 = V2γ-1Let the gas be monatomic. Then,
γ = 53
⇒V123 = V223⇒V1 = V2If this condition is met, then the two states can be connected by an isothermal as well as an adiabatic process.
Question 10:
The ratio Cp / Cv for a gas is 1.29. What is the degree of freedom of the molecules of this gas?
Answer:
For the molecules of a gas,
γ=CpCv=1+2f,
where f is the degree of freedom.
Given: γ=1.29⇒1+2f=1.29=97⇒2f=97-1⇒2f=27⇒f=7Therefore, the molecules of this gas have 7 degrees of freedom.
But in reality, no gas can have more than 6 degrees of freedom.
Question 1:
Work done by a sample of an ideal gas in a process A is double the work done in another process B. The temperature rises through the same amount in the two processes. If CA and CB be the molar heat capacities for the two processes,
(a) CA = CB
(b) CA < CB
(c) CA > CB
(d) CA and CB cannot be defined.
Answer:
(c) CA > CB
According to the first law of thermodynamics,
ΔQ=ΔU+ΔW, where
∆Q is the heat supplied to the system when
ΔW work is done on the system and
∆U is the change in internal energy produced. Since the temperature rises by the same amount in both processes, change in internal energies are same, i.e.
∆UA =
∆UB.
But as,
∆WA =
∆WB this gives
ΔQA = 2
ΔQB.
Now, molar heat capacity of a gas,
C=ΔQn ΔT, where
∆Q/n is the heat supplied to a mole of gas and
∆T is the change in temperature produced. As
∆QA = 2
∆QB,CA > CB.
Question 2:
For a solid with a small expansion coefficient,
(a) Cp − Cv = R
(b) Cp = Cv
(c) Cp is slightly greater than Cv
(d) Cp is slightly less than Cv
Answer:
c) Cp is slightly greater than Cv
For a solid with a small expansion coefficient, work done in a process will also be small. Thus, the specific heat depends slightly on the process. Therefore, Cp is slightly greater than Cv.
Question 3:
The value of Cp − Cv is 1.00 R for a gas sample in state A and 1.08 R in state B. Let pA and pB denote the pressures and TA and TB denote the temperatures of the states A and B, respectively. It is most likely that
(a) pA < pB and TA > TB
(b) pA > pB and TA < TB
(c) pA = pB and TA < TB
(d) pA > pB and TA = TB
Answer:
(a) pA < pB and TA > TB
Cp − Cv = R for the gas in state A, which means it is acting as an ideal gas in that state, whereas Cp − Cv = 1.08R in state B, i.e. the behaviour of the gas is that of a real gas in that state. To be an ideal gas, a real gas at STP should be at a very high temperature and low pressure. Therefore, PA < PB and TA > TB where PA and PB denotes the pressure and TA and TB denotes the temperature of system A and B reepectively.
Question 4:
Let Cv and Cp denote the molar heat capacities of an ideal gas at constant volume and constant pressure respectively. Which of the following is a universal constant?
(a)
CpCv(b) CpCv
(c) Cp − Cv
(d) Cp + Cv
Answer:
(c) Cp − Cv
For an ideal gas, Cp − Cv = R , where Cv and Cp denote the molar heat capacities of an ideal gas at constant volume and constant pressure, respectively and R is the gas constant whos value is 8.314 J/K. Therefore, Cp − Cv is a constant. On the other hand, the ratio of these two varies as the atomicity of the gas changes. Also, their sum and product are not constant.
Question 5:
70 calories of heat are required to raise the temperature of 2 mole of an ideal gas at constant pressure from 30° C to 35° C. The amount of heat required to raise the temperature of the same gas through the same range at constant volume is
(a) 30 calories
(b) 50 calories
(c) 70 calories
(d) 90 calories
Figure
Answer:
(b) 50 calories
It is given that 70 calories of heat are required to raise the temperature of 2 mole of an ideal gas at constant pressure from 30° C to 35° C. Also, specific heat at constant pressure,
Cp=∆Qn ∆T⇒Cp=702×(35-30)⇒Cp=702×5⇒Cp=7 calories-mol-1K-1
For an ideal gas,
Cp-Cv=R=8.314 J-mol-1K-1≃2 calories mol-1K-1⇒Cv=Cp-R⇒Cv=(7-2) calories-mol-1K-1⇒Cv=5 calories-mol-1K-1⇒Cv=∆Qn ∆T⇒5=∆Q2×(35-30)⇒∆Q=5×2×(35-30)⇒∆Q=5×2×5⇒∆Q=50 calories
Therefore, 50 calories need to be supplied to raise the temperature of 2 moles of gas from 30-35 oC at constant volume.
Question 6:
The figure shows a process on a gas in which pressure and volume both change. The molar heat capacity for this process is C.
(a) C = 0
(b) C = Cv
(c) C > Cv
(d) C < Cv
Answer:
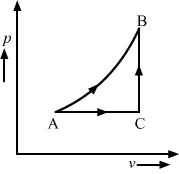
(c) C > C​v
Consider two processes AB and ACB; let W be the work done. C is the molar heat capacity of process AB. Process ACB can be considered as the sum of the two processes, AC and CB. The molar heat capacity of process AC is Cp, as pressure is constant in this process and the molar heat capacity of process CB is Cv, as volume is constant in it.
Internal energy, U, is a state function, i.e. it doesn’t depend on the path followed. Therefore,
UAB = UACBWAB>WACBWork done in the p-V diagram is the area enclosed under the curve.
⇒WAB+UAB>WACB+UACB⇒C>Cv+CpMolar heat capacity is the heat supplied per mole to change the temperature by a degree Kelvin and according to the first law of thermodynamics, dQ = dU + dW, where dQ is the heat supplied to the system in a process.
⇒C>Cv​
Question 7:
The molar heat capacity for the process shown in the figure is
(a) C = Cp
(b) C = Cv
(c) C > Cv
(d) C = 0.
Figure
Answer:
(d) C = 0.
The defined process is
p=kVg⇒pVg=k,such that the process is adiabatic in which there’s no heat supplied to the system, i.e. Q = 0. Molar heat capacity is the amount of heat supplied to the system per mole to produce a degree change in temperature. Also, in an adiabatic process, no heat exchange is allowed. So, molar heat capacity equals zero, i.e. C = 0.
Question 8:
In an isothermal process on an ideal gas, the pressure increases by 0.5%. The volume decreases by about
(a) 0.25%
(b) 0.5%
(c) 0.7%
(d) 1%.
Answer:
(b) 0.5%
Let p and p’ be the initial and final pressures of the system and V and V’ be the initial and final volumes of the system. p’ is 0.5% more than p and the process is isothernal. So, pV = k = p’V’ = constant. Therefore,
pV=p’V’⇒pV=p+0.5100pV⇒pV=100.5100pV’⇒V’=100100.5V⇒V’-V = 100100.5V-V=-0.5100.5=-0.49%So, volume V’ decreases by about 0.5% of V.
Question 9:
In an adiabatic process on a gas with γ = 1.4, the pressure is increased by 0.5%. The volume decreases by about
(a) 0.36%
(b) 0.5%
(c) 0.7%
(d) 1%
Answer:
(a) 0.36 %
Let p and p, be the initial and final pressures of the system and V and V, be the initial and final volumes of the system. p, is 0.5% more than p and the process is adiabatic. So,
pVγ=p’V’γ, γ=1.4=75⇒V’γVγ=pp’⇒V’Vγ=pp+0.5p100⇒V’V75=100100.5⇒V’V=100100.557=(200201)57⇒V’V=0.99644⇒V’=0.99644V⇒V’=V-0.00356V
Therefore, V, is 0.36 % less than V.
Question 10:
Two samples A and B are initially kept in the same state. Sample A is expanded through an adiabatic process and the sample B through an isothermal process. The final volumes of the samples are the same. The final pressures in A and B are pA and pB respectively.
(a) pA > pB
(b) pA = pB
(c) pA < pB
(d) The relation between pA and pB cannot be deduced.
Answer:
(c) pA < pB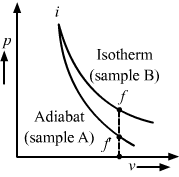
Let the initial states of samples A and B be i and the final states of samples B and A be f and f’, respectively. Let the final volumes of both be Vo. As sample A is expanded through an adiabatic process, its curve in the p-V diagram is steeper than that of sample B, which is expanded through an isothermal process. Therefore, from the p-V diagram, pA < pB.
Question 11:
Let Ta and Tb be the final temperatures of the samples A and B, respectively, in the previous question.
(a) Ta < Tb
(b) Ta = Tb
(c) Ta > Tb
(d) The relation between Ta and Tb cannot be deduced.
Answer:
(a) Ta < Tb
As sample B is undergoing expansion through an isothermal process, its initial and final temperatures will be same, i.e. Tb. On the other hand, sample A is at the same initial state as B, such that the initial temperature of A is ​Tb and it is expanding through an adiabatic process in which no heat is supplied. Therefore, sample A will expand at the cost of its internal energy and its final temperature will be less than its initial temperature.
This implies that Ta < Tb.
Question 12:
Let ∆Wa and ∆Wb be the work done by the systems A and B, respectively, in the previous question.
(a) ∆Wa > ∆Wb
(b) ∆Wa = ∆Wb
(c) ∆Wa < ∆Wb
(d) The relation between ∆Wa and ∆Wb cannot be deduced.
Answer:
(c) ∆Wa < ∆Wb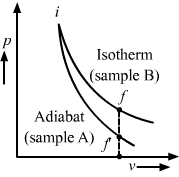
In the p-V diagram, the area under the curve w.r.t the V axis is equal to the work done by the system. Since the area under the isotherm is greater than that under the adiabat, the work done by system A is less than that done by system B. Hence, ∆Wa < ∆Wb.
Page No 77:
Question 13:
The molar heat capacity of oxygen gas at STP is nearly 2.5 R. As the temperature is increased, it gradually increases and approaches 3.5 R. The most appropriate reason for this behaviour is that at high temperatures
(a) oxygen does not behave as an ideal gas
(b) oxygen molecules dissociate in atoms
(c) the molecules collide more frequently
(d) molecular vibrations gradually become effective
Answer:
(d) molecular vibrations gradually become effective
Molar specific heat capacity has direct dependence on the degree of freedom of gas molecules. As temperature is increased, the gas molecules start vibrating about their mean position, leading to change (increase) in the degree of freedom and, hence, increasing molar heat capacity.
Question 1:
A gas kept in a container of finite conductivity is suddenly compressed. The process
(a) must be very nearly adiabatic
(b) must be very nearly isothermal
(c) may be very nearly adiabatic
(d) may be very nearly isothermal
Answer:
(c) may be very nearly adiabatic
(d) may be very nearly isothermal
Due to sudden compression, the gas did not get sufficient time for heat exchange. So, no heat exchange occurred. Therefore, the process may be adiabatic. For any process to be isothermal, its temperature should remain constant, i.e. pressure and volume should change simultaneously while their product (temperature) should be constant.
Question 2:
Let Q and W denote the amount of heat given to an ideal gas and the work done by it in an isothermal process.
(a) Q = 0
(b) W = 0
(c) Q ≠ W
(d) Q = W
Answer:
(d) Q = W
In an isothermal process, temperature of the system stays constant, i.e. there’s no change in internal energy. Thus, U = 0, where U denotes the change in internal energy of the system. According to the first law of thermodynamics, heat supplied to the system is equal to the sum of change in internal energy and work done by the system, such that Q = U + W. As U = 0, Q = W.
Question 3:
Let Q and W denote the amount of heat given to an ideal gas and the work done by it in an adiabatic process.
(a) Q = 0
(b) W = 0
(c) Q = W
(d) Q ≠ W
Answer:
(a) Q = 0
(d) Q ≠ W
In an adiabatic process, no heat is supplied to the system; so, Q = 0. According to the first law of thermodynamics, heat given to any system is equal to the sum of the change in internal energy and the work done on the system. So, Q = W+U and as Q = 0, W = –U and Q ≠ W.
Question 4:
Consider the processes A and B shown in the figure. It is possible that
Figure
(a) both the processes are isothermal
(b) both the processes are adiabatic
(c) A is isothermal and B is adiabatic
(d) A is adiabatic and B is isothermal
Answer:
(c) A is isothermal and B is adiabatic
The slope of an adiabatic process is greater than that of an isothermal process. Since A and B are initiated from the same initial state, both cannot be isothermal or adiabatic, as they would be overlapping. But the curve of process B is steeper than the curve of process A. Hence, A is isothermal and B is adiabatic.
Question 5:
Three identical adiabatic containers A, B and C contain helium, neon and oxygen, respectively, at equal pressure. The gases are pushed to half their original volumes.
(a) The final temperatures in the three containers will be the same.
(b) The final pressures in the three containers will be the same.
(c) The pressures of helium and neon will be the same but that of oxygen will be different.
(d) The temperatures of helium and neon will be the same but that of oxygen will be different.
Answer:
(c) The pressures of helium and neon will be the same but that of oxygen will be different.
(d) The temperatures of helium and neon will be the same but that of oxygen will be different.
Adiabatic process is expressed as
pVγ=constant orTVγ-1=constant,
where
γ=CpCv is the ratio of molar heat capacities at constant pressure and volume.
We know that
γis equal to 1.67 and 1.40 for a monatomic gas and a diatomic gas, respectively. Helium and neon are monatomic gases and oxygen is a diatomic gas. Therefore, changing the state of the gases, i.e. reducing the volume will lead to identical changes in temperature and pressure for helium and neon and that will be different for oxygen.
Question 6:
A rigid container of negligible heat capacity contains one mole of an ideal gas. The temperature of the gas increases by 1° C if 3.0 cal of heat is added to it. The gas may be
(a) helium
(b) argon
(c) oxygen
(d) carbon dioxide
Answer:
(a) helium
(b) argon
The temperature of one mole of a gas kept in a container of fixed volume is increased by 1 degree Celsius if 3 calories, i.e. 12.54 J of heat is added to it. So, its molar heat capacity, C​v = 12.54 J
JK-1mol-1, as molar heat capacity at fixed volume is the heat supplied to a mole of gas to increase its temperature by a degree. For a monatomic gas, ​C​v
≃ 32R=1.5×8.314= 12.54 JK-1mol-1. Among the given gases, only helium and argon are inert and, hence, monoatomic. Therefore, the gas may be helium or argon.
Question 7:
Four cylinders contain equal number of moles of argon, hydrogen, nitrogen and carbon dioxide at the same temperature. The energy is minimum in
(a) argon
(b) hydrogen
(c) nitrogen
(d) carbon dioxide
Answer:
(a) argon
The energy of a gas is measured as CvT. All the four cylinders are at the same temperature but the gases in them have different values of Cv, such that it is least for the monatomic gas and keeps on increasing as we go from monatomic to tri-atomic. Among the above gases, argon is monatomic, hydrogen and nitrogen are diatomic and carbon dioxide is tri-atomic. Therefore, the energy is minimum in argon.
Question 1:
A vessel containing one mole of a monatomic ideal gas (molecular weight = 20 g mol−1) is moving on a floor at a speed of 50 m s−1. The vessel is stopped suddenly. Assuming that the mechanical energy lost has gone into the internal energy of the gas, find the rise in its temperature.
Answer:
Number of moles of the ideal gas, n = 1 mole
Molecular weight of the gas, W = 20 g/mole
Mass of the gas, m =20 g
Velocity of the vessel, V = 50 m/s
Decrease in K.E. of the vessel = Internal energy gained by the gas
KE=12m(u2-v2)KE=12×20×10-3×(0-50×50)KE=-25 J=gain in internal energy of the gasChange in internal energy of a gas=d2nR(∆T),where d is the degre of freedom of the gas For a monoatomic gas, d=3.So, 25=32 nR(∆T)⇒25=1×32×8.31×∆T⇒∆T=50(3×8.3)=2 K
Question 2:
5 g of a gas is contained in a rigid container and is heated from 15°C to 25°C. Specific heat capacity of the gas at constant volume is 0.172 cal g−1 °C−1 and the mechanical equivalent of heat is 4.2 J cal−1. Calculate the change in the internal energy of the gas.
Answer:
Given:
Mass of the gas, m = 5 g
Change in temperature of the system, ∆T = 25 − 15°C = 10°C
Specific heat at constant volume, Cv = 0.172 cal/g -°C
Mechanical equivalent, J = 4.2 J/cal
From the first law of thermodynamics,
dQ = dU + dW
Now,
∆V = 0 (Rigid wall of the container keeps the volume constant)
So, dW = P
∆V= 0
Therefore,
dQ = dU (From the first law)
Q = m
cvdT = 5 × 0.172 × 10
= 8.6 cal = 8.6 × 4.2 J
= 36.12 J
So, change in internal energy of the system is 36.12 J.
Question 3:
The figure shows a cylindrical container containing oxygen (γ = 1.4) and closed by a 50-kg frictionless piston. The area of cross-section is 100 cm2, atmospheric pressure is 100 kPa and g is 10 m s−2. The cylinder is slowly heated for some time. Find the amount of heat supplied to the gas if the piston moves out through a distance of 20 cm.
Figure
Answer:
Given:
Mass of the piston (m) = 50 kg
Adiabatic constant of the gas, γ = 1.4
Area of cross-section of the piston (A) = 100 cm2
Atmospheric pressure (P0) = 100 kPa
g = 10 m/s2
Distance moved by the piston , x = 20 cm
Work done by the gas,
dW=PdvThe pressure (p) is because of two factors: thefirst is the initial pressure and the second is the weight of the piston.Therefore, W=mgA+P0×AdxW=50×10100×10-4+105×100×10-4×20×10-4W = (5 × 104 + 105) × 20 × 10−4
W = 1.5 × 105 × 20 × 10−4
W = 300 J
Hence, nRdT = P
∆V= 300
⇒dT=300nRSo, dQ=nCpdT=ncp×300nRUsing Cp-Cv=R and CpCv=γ,dQ=nγR300(γ-1)nRdQ=300×1.40.4=1050 J
Question 4:
The specific heat capacities of hydrogen at constant volume and at constant pressure are 2.4 cal g−1 °C−1 and 3.4 cal g−1 °C−1 respectively. The molecular weight of hydrogen is 2 g mol−1 and the gas constant, R = 8.3 × 107 erg °C−1 mol−1. Calculate the value of J.
Answer:
Specific heat capacity at constant volume, Cv(H2) = 2.4 cal/g-°C
Specific heat capacity at constant pressure, Cp(H2) = 3.4 cal/g-°C
Molecular weight, M = 2 g/mol
Gas constant, R = 8.3 × 107 erg/mol-°C
We know: Cp − Cv = 1 cal/g-°C,
where Cp and Cv are molar specific heat capacities.
So, difference of molar specific heat,
Cp × M − Cv × M = 1 cal/mol-°C
Now, 2 × J = R
⇒ 2 × J = 8.3 × 107 erg/mol-°C
⇒ J = 4.15 × 107 erg/cal
Question 5:
The ratio of the molar heat capacities of an ideal gas is Cp/Cv = 7/6. Calculate the change in internal energy of 1.0 mole of the gas when its temperature is raised by 50 K (a) keeping the pressure constant (b) keeping the volume constant and (c) adiabatically.
Answer:
Given:
CpCv=76
Number of moles of the gas, n = 1 mol
Change in temperature of the gas, ∆T = 50 K
(a) Keeping the pressure constant:
Using the first law of thermodynamics,
dQ = dU + dW
∆T = 50 K and
γ=76
dQ = dU + dW
Work done, dW = PdV
As pressure is kept constant, work done = P(
∆V)
Using the ideal gas equation PV = nRT,
P(
∆V) = nR(
∆T)
⇒ dW = nR(
∆T)
At constant pressure, dQ = nCp dT
Substituting these values in the first law of thermodynamics, we get
nCp dT = dU + RdT
⇒ dU = nCp dT − RdT
Using
CpCV=γ and CP-CV=R, we get
dU=1×Rγ(γ-1)×dT-RdT = 7 RdT − RdT
= 7 RdT − RdT = 6 RdT
= 6 × 8.3 × 50 = 2490 J
(b) Keeping volume constant:
Work done = 0
Using the first law of thermodynamics,
dU = dQ
dU = nCv dT
=1×Rγ-1×dT=1×8.376-1×50= 8.3 × 50 × 6 = 2490 J
(c) Adiabatically, dQ = 0
Using the first law of thermodynamics, we get
dU = − dW
=n×Rγ-1(T1-T2)=1×8.37/6-1=(T2-T1) = 8.3 × 6 × 50 = 2490 J
Page No 78:
Question 6:
A sample of air weighing 1.18 g occupies 1.0 × 103 cm3 when kept at 300 K and 1.0 × 105 Pa. When 2.0 cal of heat is added to it at constant volume, its temperature increases by 1°C. Calculate the amount of heat needed to increase the temperature of air by 1°C at constant pressure if the mechanical equivalent of heat is 4.2 × 107 erg cal−1. Assume that air behaves as an ideal gas.
Answer:
Here,
m = 1.18 g = 1.18×10-3 kg
ΔQ = 2.0×4.2 J
P = 1.0×106 Pa
V = 1.0×103 cm3 = 1.0×10-3 m3
T = 300K
Applying eqn. of state
PV = nRT
=> n = PV/RT
=> n = 1.0×105×1.0×10-3/(8.314×300)
=> n = 0.04
ΔT = 10C
(ΔH)v = nCv ΔT
2.0×4.2 = nCv×1
=> Cv = 8.4/n= 8.4/0.04
=> Cv = 210
Again we know
Cp – Cv =R
=> Cp = R + Cv
=> Cp = 8.3 + 210
=> Cp = 218.3
Now at constant pressure
(ΔH)p = nCp ΔT
=> (ΔH)p = 218.3×0.04×1 = 8.732 J
In calories
=> (ΔH)p = 8.732/4.2 = 2.08 cal
Question 7:
An ideal gas expands from 100 cm3 to 200 cm3 at a constant pressure of 2.0 × 105 Pa when 50 J of heat is supplied to it. Calculate (a) the change in internal energy of the gas (b) the number of moles in the gas if the initial temperature is 300 K (c) the molar heat capacity Cp at constant pressure and (d) the molar heat capacity Cv at constant volume.
Answer:
Initial volume of the gas, V1 = 100 cm3
Final volume = V2 = 200 cm3
Pressure = 2 × 105 Pa
Heat supplied, dQ = 50 J
(a) According to the first law of thermodynamics,
dQ = dU + dW
dW = P
∆V=2×105×(200-100)×10-6=20⇒ 50 = dU + 2 × 10
⇒ dU = 30 J
(b) For a monatomic gas,
U =
32nRT
30 = n ×
32× 8.3 × 300
⇒n=2(83×3)=2249=0.008(c) Also,
dU = nCvdT
⇒Cv=dUndT=300.008×300=12.5Cp = Cv + R = 12.5 + 8.3 = 20.8 J/mol-K
(d) Cv = 12.5 J/mol-K
Question 8:
An amount Q of heat is added to a monatomic ideal gas in a process in which the gas performs a work Q/2 on its surrounding. Find the molar heat capacity for the process.
Answer:
Given:
Amount of heat given to the gas = Q
So, ∆Q = Q
Work done by the gas, ∆W =
Q2
From the first law of thermodynamics,
∆Q = ∆W + ∆U
⇒∆U=Q-Q2=Q2For a monoatomic gas,
∆U =
32nRdT
⇒Q2=ndT×32R
⇒Q = 3nRdT
Again, for expansion at constant pressure,
Q = nCpdT,
where Cp is the molar heat capacity at constant pressure.
So, 3RndT = nCpdT
⇒ Cp = 3R
Question 9:
An ideal gas is taken through a process in which the pressure and the volume are changed according to the equation p = kV. Show that the molar heat capacity of the gas for the process is given by C = Cv +
R2.
Answer:
Relation between pressure and volume of a gas is P = kV.
Ideal gas equation is PV = nRT.
⇒nRTV=kV ⇒nRT=kV2
For simplicity, take the number of moles of a gas, n = 1.
⇒ RdT = 2 kVdV
⇒RdT2kV=dV
From the first law of thermodynamics,
dQ = dU + dW
⇒ n
CpdT = Cv dT + PdV
⇒nCpdT = Cv dT+PRdT2kV⇒1×Cp= Cv+PR2kV∴ Cp=Cv+R2
Question 10:
An ideal gas (Cp / Cv = γ) is taken through a process in which the pressure and the volume vary as p = aVb. Find the value of b for which the specific heat capacity in the process is zero.
Answer:
As the process has specific heat capacity zero, the process is essentially an adiabatic process.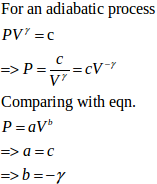
Question 11:
Two ideal gases have the same value of Cp / Cv = γ. What will be the value of this ratio for a mixture of the two gases in the ratio 1 : 2?
Answer:
For the first ideal gas,
Cp1 = specific heat at constant pressure
Cv1 = specific heat at constant volume
n1 = number of moles of the gas
Cp1CV1= γ and Cp1 − Cv1 = R
⇒ γCv1 − Cv1 = R
⇒ Cv1 (γ − 1) = R
⇒Cv1=R(γ-1)Cp1=γR(γ-1)For the second ideal gas,
Cp2 = specific heat at constant pressure
Cv2 = specific heat at constant volume
n2 = number of moles of the gas
Cp2Cv2= γ and Cp2 − Cv2 = R
⇒ γCv2 − Cv2 = R
⇒ Cv2 (γ − 1) = R
⇒Cv2=R(γ-1) andCp2=γR(γ-1)Given:
n1 = n2 = 1 : 2
dU1 = nCv1dt
dU2= 2nCv2dT
When the gases are mixed,
nCv1dT + 2nCv2dT = 3nCvdT
Cv=Cv1+2Cv23=R(γ-1)+2R(γ-1)3=3R(γ-1)3=Rγ-1Hence, Cp / Cv in the mixture is γ.
Question 12:
A mixture contains 1 mole of helium (Cp = 2.5 R, Cv = 1.5 R) and 1 mole of hydrogen (Cp = 3.5 R, Cv = 2.5 R). Calculate the values of Cp, Cv and γ for the mixture.
Answer:
Specific heat at constant pressure of helium, Cp‘ = 2.5 R
Specific heat at constant pressure of hydrogen, Cp“ = 3.5 R
Specific heat at constant volume of helium, Cv‘ = 1.5 R
Specific heat at constant volume of hydrogen, Cv“ = 2.5 R
n1 = n2 = 1 mol
dU=nCvdT
For the mixture of two gases,
dU1+dU2=1 mol[n1 + n2] CvdT = n1C’vdT + n2C“vdT,
where
CVis the heat capacity of the mixture.
⇒Cv=n1C’v+n2C”vn1+n2=1.5R+2.5R2=2RCp = Cv + R = 2R + R = 3R
γ=CpCv=3R2R=1.5
Question 13:
Half mole of an ideal gas (γ = 5/3) is taken through the cycle abcda, as shown in the figure. Take
R=253J K-1 mol-1. (a) Find the temperature of the gas in the states a, b, c and d. (b) Find the amount of heat supplied in the processes ab and bc. (c) Find the amount of heat liberated in the processes cd and da.
Figure
Answer:
Given:
Number of moles of the gas,
n=0.5 mol
R=253 J/mol-Kγ=53(a) Temperature at a =
Ta
PaVa=nRTa
⇒Ta=PaVanR=100×103×5000×10-60.5×253=120 KSimilarly, temperature at b,
Tb=PbVbnRTb=100×103×10000×10-60.5×253Tb=240 K Similarly, temperature at c is 480 K and at d is 240 K.
(b) For process ab,
dQ = ncpdT
[Since ab is isobaric]
dQ=12×Rγγ-1(Tb-Ta)dQ=12×25×53×353-1×(240-120)dQ=12×1259×32×(120)dQ=1250 JFor line bc, volume is constant. So, it is an isochoric process.
dQ = dU + dW
[dW = 0, isochoric process]
dQ = dU = nCvdT
dQ= nCv (
Tc-Tb)
dQ=12×25353-1×(240)dQ=12×253×32×240=1500 J(c) Heat liberated in cd (isobaric process),
dQ = − nCpdT
dQ=-12×γR(γ-1)×(Td-Tc)dQ=-12×1259×32×(240-480)dQ=-12×1256×240=2500 JHeat liberated in da (isochoric process),
⇒dQ = dU
Q= −nCvdT
dQ=-12×Rγ-1Ta-TddQ=-12×252×(120-240)dQ=254×120=750 J
Question 14:
An ideal gas (γ = 1.67) is taken through the process abc shown in the figure. The temperature at point a is 300 K. Calculate (a) the temperatures at b and c (b) the work done in the process (c) the amount of heat supplied in the path ab and in the path bc and (d) the change in the internal energy of the gas in the process.
Figure
Answer:
(a) For line ab, volume is constant.
So, from the ideal gas equation,
P1T1=P2T2⇒100300=200T2
T2=200×300100=600 KFor line bc, pressure is constant.
So, V1T1=V2T2⇒100600=150T2⇒T2 600×150100=900 K(b)
As process ab is isochoric,
Wab=0.
During process bc,
P = 200 kPa
The volume is changing from 100 to 150 cm3 .
Therefore, work done = 50 × 10−6 × 200 × 103 J
= 10 J
(c) For ab (isochoric process), work done = 0.
From the first law,
dQ = dU = nCvdT
⇒Heat supplied = nCvdT
Now,
Qab=PVRT×Rγ-1×dT=200×103×100×10-6×300600×0.67=14.925 (∴γ=1.67)For bc (isobaric process):Heat supplied in bc=nCpdT Cp=γRγ-1=PVRT×γRγ-1×dT=200×103×150×10-6600×0.67×300=10×1.670.67=16.70.67=24.925(d) dQ = dU + dW
Now,
dU = dQ − dW
= Heat supplied − Work done
= (24.925 + 14.925) − 10
= 39.850 − 10 = 29.850 J.
Question 15:
In Joly’s differential steam calorimeter, 3 g of an ideal gas is contained in a rigid closed sphere at 20°C. The sphere is heated by steam at 100°C and it is found that an extra 0.095 g of steam has condensed into water as the temperature of the gas becomes constant. Calculate the specific heat capacity of the gas in J g−1 K−1. The latent heat of vaporisation of water = 540 cal g−1
Answer:
For Joly’s differential steam calorimeter,
Cv=m2Lm1 (θ2−θ1),
where
m2 = mass of steam condensed
m2 = 0.095 g
Latent heat of vapourization, L = 540 cal/g = 540 × 4.2 J/g
m1 = mass of gas present
m1 = 3 g
Initial temperature, θ1 = 20°C
Final temperature, θ2 = 100°C
⇒Cv=0.095×540×4.23×(100-20) = 0.89 = 0.9 J/g-K
Question 16:
The volume of an ideal gas (γ = 1.5) is changed adiabatically from 4.00 litres to 3.00 litres. Find the ratio of (a) the final pressure to the initial pressure and (b) the final temperature to the initial temperature.
Answer:
Given,
γ = 1.5
Since the process is adiabatic, PVγ = constant.
(a) P1V1γ = P2V2γ
Given, V1 = 4 L
V2 = 3 L
We need to find
P2P1.
⇒P2P1=V1V2γ=431.5=1.5396=1.54(b) Also, for an adiabatic process,
TVγ−1 = constant
T1V1γ−1 = T2V2γ−1
⇒T2T1=V1V2γ-1=430.5=1.154
Question 17:
An ideal gas at pressure 2.5 × 105 Pa and temperature 300 K occupies 100 cc. It is adiabatically compressed to half its original volume. Calculate (a) the final pressure (b) the final temperature and (c) the work done by the gas in the process. Take γ = 1.5
Answer:
Initial pressure of the gas, P1 = 2.5 × 105 Pa
Initial temperature, T1 = 300 K
Initial volume, V1 = 100 cc
(a) For an adiabatic process,
P1V1γ = P2V2γ
⇒ 2.5 × 105 × V1.5 =
V21.5× P2
⇒ P2 = 7.07 × 105
= 7.1 × 105 Pa
(b) Also, for an adiabatic process,
T1V1γ−1 = T2V2γ−1
⇒ 300 × (100)1.5−1 = T2 ×
10021.5-1= T2 × (50)1.5−1
⇒ 300 × 10 = T2 × 7.07
⇒ T2 = 424.32 K = 424 K
(c) Work done by the gas in the process,
W=nR(γ-1) [T1-T2]=P1V1T(γ-1) [T1-T2]=2.5×10300×0.5×(-124)= -20.67 ≈ -21 J
Question 18:
Air (γ = 1.4) is pumped at 2 atm pressure in a motor tyre at 20°C. If the tyre suddenly bursts, what would be the temperature of the air coming out of the tyre? Neglect any mixing with the atmospheric air.
Answer:
Given:
For air, γ = 1.4
Initial temperature of air, T1 = 20°C = 293 K
Initial pressure, P1 = 2 atm
Final pressure, P2 = 1 atm
The bursting of the tyre is an adiabatic process. For an adiabatic process,
P11−γ × T11−γ = P1−γ × T2γ
(2)1−1.4 × (293)1.4 = (1)1−1.4 × T21.4
⇒ (2)−0.4 × (293)1.4 = T21.4
⇒ 2153.78 = T21.4
⇒ T2 = (2153.78)1/1.4
= 240.3 K
Question 19:
A gas is enclosed in a cylindrical can fitted with a piston. The walls of the can and the piston are adiabatic. The initial pressure, volume and temperature of the gas are 100 kPa, 400 cm3 and 300 K, respectively. The ratio of the specific heat capacities of the gas, Cp / Cv = 1.5. Find the pressure and the temperature of the gas if it is (a) suddenly compressed (b) slowly compressed to 100 cm3.
Answer:
Initial pressure of the gas, P1 = 100 kPa
Initial volume of the gas,V1 = 400 cm3
= 400 × 10−6 m3
Initial temperature of the gas, T1 = 300 K
γ=CpCv=1.5
(a) The gas is suddenly compressed to volume, V2 = 100 cm3 .
So, this is an adiabatic process.
For an adiabatic process,
P1V1γ = P2V2γ
⇒ 105 × (400)1.5 = P2 (100)1.5
⇒ P2 = 105(4)1.5 = 800 kPa
Also,
T1Vγ−1 = T2V2γ−1
⇒ 300 × (400)1.5−1 = T2 (100)1.5−1
⇒ 300 × (400)0.5 = T2 (100)0.5
⇒ T2 = 600 K
(b) If the container is slowly compressed, the heat transfer is zero, even thought the walls are adiabatic.
Thus, the values remain same. Thus,
P2 = 800 kPa
T2 = 600 K
Question 20:
The initial pressure and volume of a given mass of a gas (Cp/Cv = γ) are p0 and V0. The gas can exchange heat with the surrounding. (a) It is slowly compressed to a volume V0/2 and then suddenly compressed to V0/4. Find the final pressure. (b) If the gas is suddenly compressed from the volume V0 to V0/2 and then slowly compressed to V0/4, what will be the final pressure?
Answer:
Given:
For the gas,
CpCv=γ
Initial pressure of the gas = P0
Initial volume of the gas = V0
(a)
(i) As the gas is slowly compressed, its temperature will remain constant.
For isothermal compression,
P1V1 = P2V2
So, P0V0 = P2
V02⇒ P2 = 2P0
(ii) Sudden compression means that the gas could not get sufficient time to exchange heat with its surroundings. So, it is an adiabatiac compression.
So, for adiabatic compression,
P1V1γ = P2V2γ
Or 2P0V02γ=P2V04γ⇒P2=V0γ2γ×2P0×4γV0γ= 2γ × 2P0 = P02γ+1
(b)
(i) Adiabatic compression:
P1V1γ = P2V2γ
P0V0γ = P’
V02γ
⇒ P’ = P02γ
(ii) Isothermal compression:
P1V1 = P2V2
2γP0×V02=P”V02P” = P02γ × 2
⇒ P” = P02γ+1
Question 21:
Consider a given sample of an ideal gas (Cp/Cv = γ) having initial pressure p0 and volume V0. (a) The gas is isothermally taken to a pressure p0/2 and from there, adiabatically to a pressure p0/4. Find the final volume. (b) The gas is brought back to its initial state. It is adiabatically taken to a pressure p0/2 and from there, isothermally to a pressure p0/4. Find the final volume.
Answer:
(a) Given,
Initial pressure of the gas = p0
Initial volume of the gas =
V0
For an isothermal process,
PV = constant
So, P1V1 = P2V2
P2=P0V0(P02)=2V0
For an adiabatic process, P3 =
P04, V3 = ?
P2V2γ = P3V3γ
⇒V3V2γ=P2P3⇒V3V2γ=P02P04=2⇒V3V2=21γ∴ V3=V2 21γ=2V0 21γ=2γ+1γV0(b) P1V1γ = P2V2γ
Or V2V1=P1P21γ⇒ V2=V0 21γ
Again, for an isothermal process,
P2V2 = P3V3
∴ V3=P2V2P3=221γV0=2γ+1γV0
Question 22:
A sample of an ideal gas (γ = 1.5) is compressed adiabatically from a volume of 150 cm3 to 50 cm3. The initial pressure and the initial temperature are 150 kPa and 300 K. Find (a) the number of moles of the gas in the sample (b) the molar heat capacity at constant volume (c) the final pressure and temperature (d) the work done by the gas in the process and (e) the change in internal energy of the gas.
Answer:
The ideal gas equation is
PV = nRT
Given,
P1= 150 kPa = 150 × 103 Pa
V1 = 150 cm3 = 150 × 10−6 m3
T1= 300 K
(a)
n=PVRT=9.036×10-3n=0.009(b)
CpCv=γ, Cp-Cv=RSo, Cv=Rγ-1=2R=8.30.5=16.6 J/mole-K(c) Given,
P1 = 150 kPa = 150 × 103 Pa
P2 = ? V1 = 150 cm3
= 150 × 10−6 m3
γ = 1.5
V2 = 50 cm3 = 50 × 10−6 m3,
T1 = 300 K
T2 = ?
Since the process is adiabatic, using the equation of an adiabatic process,we get
P1V1γ = P2V2γ
⇒ 150 × 103 × (150 × 10−6)γ = P2 × (50 × 10−6)γ
⇒P2=150×103×(150×10-6)1.5(50×10-6)1.5P2 = 150000 × (3)1.5
P2 = 779.422 × 103 Pa
P2 = 780 kPa
Again,
P11−γ T1γ = P11−γ T2γ
⇒ (150 × 103)1−1.5 × (330)1.5 = (780 × 103)1−1.5 × T21.5
⇒ T21.5 = (150 × 103)1−1.5 × (300)1.5 × 3001.5
T21.5 = 11849.050
⇒ T2 = (11849.050)1/1.5
T2 = 519.74 = 520 K
(d) dQ = dW + dU
Or dW = −dU [ Since dQ = 0 in an adiabatic process]
dW = −nCvdT
dW = −0.009 × 16.6 × (520 − 300)
dW = −0.009 × 16.6 × 220
dW = −32.87 J
≈−33 J
(e)
dU = nCvdT
dU = 0.009 × 16.6 × 220
≈33 J
Page No 79:
Question 23:
Three samples A, B and C of the same gas (γ = 1.5) have equal volumes and temperatures. The volume of each sample is doubled, the process being isothermal for A, adiabatic for B and isobaric for C. If the final pressures are equal for the three samples, find the ratio of the initial pressures.
Answer:
There are three gases A, B and C.
It is given that initially,
VA = VB = VCandTA = TB = TC .
For A, the process is isothermal and for an isothermal process, PV = constant.
PAVA = P’A2VA
⇒P’A=PA×12
For B, the process is adiabatic. So,
PBVBγ = PB‘(2VB)γ
⇒PB’=PB21.5For C, the process is isobaric, which implies that the pressure will remain constant.
So, using the ideal gas equation
P
=nRTV, we get
VCTC=V’CT’C⇒VCTC=2VCT’C⇒ TC‘ = 2TC
As the final pressures are equal,
PA2=PB21.5=PC⇒PA : PB : PC=2 : 21.5 : 1
Question 24:
Two samples A and B, of the same gas have equal volumes and pressures. The gas in sample A is expanded isothermally to double its volume and the gas in B is expanded adiabatically to double its volume. If the work done by the gas is the same for the two cases, show that γ satisfies the equation 1 − 21−γ = (γ − 1) ln2.
Answer:
Let,
Initial pressure of the gas = P1
Initial volume of the gas = V1
Final pressure of the gas= P2
Final volume of the gas = V2
Given, V2 = 2 V1, for each case.
In an isothermal expansion process,
work done
=nRT1 lnV2V1Adiabatic work done,
W=P1V1-P2V2γ-1It is given that same work is done in both cases.
So,
nRT1 ln(V2V1)=P1V1-P2V2γ-1 …(1)In an adiabatic process,
P2=P1V1V2γ=P112γFrom eq (1),
nRT1 ln 2=P1V11-12γ×2γ-1and nRT1 = P1V1
So, ln 2=1-12γ.2γ-1Or (γ − 1) ln 2 = 1 − 21−γ
Question 25:
1 litre of an ideal gas (γ = 1.5) at 300 K is suddenly compressed to half its original volume. (a) Find the ratio of the final pressure to the initial pressure. (b) If the original pressure is 100 kPa, find the work done by the gas in the process. (c) What is the change in internal energy? (d) What is the final temperature? (e) The gas is now cooled to 300 K keeping its pressure constant. Calculate the work done during the process. (f) The gas is now expanded isothermally to achieve its original volume of 1 litre. Calculate the work done by the gas. (g) Calculate the total work done in the cycle.
Answer:
Given:
γ = 1.5
T = 300 K
Initial volume of the gas, V1 = 1 L
Final volume, V2 =
12L
(a) The process is adiabatic because volume is suddenly changed; so, no heat exchange is allowed.
P1V1γ = P2V2γ
Or P2=P1V1V2γ=P1(2)γ P2P1=21.5=22(b) P1 = 100 kPa = 105 Pa
and P2 =
22× 105 Pa
Work done by an adiabatic process,
W=P1V1-P2V2γ-1W=105×10-3-22×105×12×10-31.5-1W=-82 J(c) Internal energy,
dQ = 0, as it is an adiabatic process.
⇒ dU = − dW = − (− 82 J) = 82 J
(d)
Also, for an adiabatic process,
T1V1γ−1 = T2V2γ−1
T2 = T1
V1V2γ-1= 300
×(2)0.5
= 300 ×
2× = 300 × 1.4142
T2 = 424 K
(e) The pressure is kept constant.
The process is isobaric; so, work done = P
∆V=nRdT.
Here,
n=PVRT=105×10-3R×300=13RSo, work done =
13R×R×(300-424)=-41.4 JAs pressure is constant,
V1T1=V2T2 …(1)V1=V2T1T2(f)Work done in an isothermal process,
W=nRT lnV2V1=13R×R×T×In (2)= 100 × ln 2 = 100 × 1.039
= 103 J
(g) Net work done (using first law of thermodynamics)
= − 82 − 41.4 + 103
= − 20.4 J
Question 26:
Figure shows a cylindrical tube with adiabatic walls and fitted with an adiabatic separator. The separator can be slid into the tube by an external mechanism. An ideal gas (γ = 1.5) is injected in the two sides at equal pressures and temperatures. The separator remains in equilibrium at the middle. It is now slid to a position where it divides the tube in the ratio 1 : 3. Find the ratio of the temperatures in the two parts of the vessel.
Figure
Answer:
Given:
γ = 1.5
For an adiabatic process, TVγ−1 = constant .
So, T1 V1γ−1 = T2 V2γ−1
As it is an adiabatic process and all the other conditions are same, the above equation can be applied.
In the new position, the slid is dividing the tube in the ratio 3:1.
So, if the total volume is V, then one side will occupy a volume of
34Vand the other side will occupy
V4.
So,
T1×3v4γ-1=T2×v4γ-1(i) 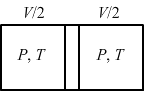 (ii)
(ii) 
⇒T1×3v41.5-1=T2×v41.5-1⇒T1T2=31
Question 27:
Figure shows two rigid vessels A and B, each of volume 200 cm3, containing an ideal gas (Cv = 12.5 J K−1 mol−1). The vessels are connected to a manometer tube containing mercury. The pressure in both the vessels is 75 cm of mercury and the temperature is 300 K. (a) Find the number of moles of the gas in each vessel. (b) 5.0 J of heat is supplied to the gas in vessel A and 10 J to the gas in vessel B. Assuming there’s no appreciable transfer of heat from A to B, calculate the difference in the heights of mercury in the two sides of the manometer. Gas constant, R = 8.3 J K−1 mol−1.
Figure
Answer:
Given:
Volume of gas in each vessel, V = 200 cm3
Specific heat at constant volume of the gas, Cv = 12.5 J/mol-K
Initial temperature of the gas, T = 300 K
Initial pressure of the gas, P = 75 cm of Hg
(a) Using the ideal gas equation, number of moles of gases in each vessel,
75 cm of Hg = 99991.5 N/m2n=PVRT=99991.5×200×10-68.3×300=8031.4×10-6=0.008(b) Heat is supplied to the gas, but dV is zero as the container has rigid walls.
So, dW =
P∆V= 0
From first law of thermodynamics,
dQ = dU
⇒ 5 = nCvdT
⇒ 5 = 0.008 × 12.5 × dT
⇒ dT = 50 for A
PT=PATAbecause volume is kept constant.
Q=nCvdTT=QnCv ⇒75300=PA×0.008×12.55⇒PA=75×5300×0.008×12.5 =12.5 cm of HgAgain, PT=PBTB [For container B]⇒75300=PB ×0.008×12.510PB = 25 cm of Hg
The distance moved by the mercury,
PB − PA = 25 − 12.5 = 12.5 cm
Question 28:
The figure shows two vessels with adiabatic walls, one containing 0.1 g of helium (γ = 1.67, M = 4 g mol−1) and the other containing some amount of hydrogen (γ = 1.4, M = 2 g mol−1). Initially, the temperatures of the two gases are equal. The gases are electrically heated for some time during which equal amounts of heat are given to the two gases. It is found that the temperatures rise through the same amount in the two vessels. Calculate the mass of hydrogen.
Figure
Answer:
Given:
Mass of He, mHe = 0.1 g
γ1 = 1.67
Molecular weight of He, MHe = 4 g/mol
MH2 = ?
MH2 = 2 g/mol
γ2 = 1.4
Since it is an adiabatic environment and the system is not dong any external work, the amount of heat given will be used up entirely to raise its internal energy.
For He, dQ = dU = nCvdT …(i)
=m4×Rγ-1×dT=0.14×R(1.67-1)×dTFor H2, dQ =dU = nCvdT …(ii)
=m2×Rγ-1×dT=m2×R1.4-1×dT,where m is the required mass of H2.
Since equal amount of heat is given to both gases; so dQ is same in both eq (i) and (ii), we get
0.14×R0.67dT=m2×R0.4×dT⇒m=0.12×0.40.67⇒m=0.0298≈0.03 g
Question 29:
Two vessels A and B of equal volume V0 are connected by a narrow tube that can be closed by a valve. The vessels are fitted with pistons that can be moved to change the volumes. Initially, the valve is open and the vessels contain an ideal gas (Cp/Cv = γ) at atmospheric pressure p0 and atmospheric temperature T0. The walls of vessel A are diathermic and those of B are adiabatic. The valve is now closed and the pistons are slowly pulled out to increase the volumes of the vessels to double the original value. (a) Find the temperatures and pressures in the two vessels. (b) The valve is now opened for sufficient time so that the gases acquire a common temperature and pressure. Find the new values of the temperature and pressure.
Answer:
Initial pressure of the gas in both the vessels = P0
Initial temperature of the gas in both the vessels = T0
Initial volume = V0
CpCv=γ
(a) The temperature inside the diathermic vessel remains constant. Thus,
P1V1 = P2V2
⇒ P0V0 = P2 × 2V0
⇒P2=P02
Temperature = T0
The temperature inside the adiabatic vessel does not remain constant.
So, for an adiabatic process,
T1V1γ−1 = T2V2γ−1
⇒ T0V0γ−1 = T × (2V0)γ−1
⇒ T2 = T0 × 21−γ
⇒ P1V1γ = P2V2γ
P0V0γ = P2 × (2V0)γ
⇒P2=P02γ
(b) When the valves are open, the temperature remains T0 throughout, i.e. T1 = T2 = T0 .
Also, pressure will be same throughout. Thus, P1 = P2. As, temperature has not changed on side 1, so pressure on this side will also not change (volume is also fixed due to fixed piston) and will be equal to
P0(pressure is an intrinsic variable).
On side 2, pressure will change to accommodate the changes in temperature on this side.
So, P0 = P1 + P2
⇒ 2P1 = 2P2
So, P1 = P2 =
P02
Question 30:
The figure shows an adiabatic cylindrical tube of volume V0 divided in two parts by a frictionless adiabatic separator. Initially, the separator is kept in the middle, an ideal gas at pressure p1 and temperature T1 is injected into the left part and another ideal gas at pressure p2 and temperature T2 is injected into the right part. Cp/Cv = γ is the same for both the gases. The separator is slid slowly and is released at a position where it can stay in equilibrium. Find (a) the volumes of the two parts (b) the heat given to the gas in the left part and (c) the final common pressure of the gases.
Figure
Answer:
For an adiabatic process, PVγ = Constant
So, P1V1γ = P2V2γ …(i)
According to the problem,
V1 + V2 = V0 …(ii)
Using the relation in eq (ii) in eq (i), we get
P1V1γ = P2(V0 − V1)γ
Or P1P21/γ=V0-V1V1 V1P11γ=V0P21γ-V1P21γV1P11γ+P21γ=V0P21γ V1=P21γV0P11γ+P21γ
Using equation (ii), we get
V2=P11γV0P11γ+P21γ
(b) Since the whole process takes place in adiabatic surroundings, the separator is adiabatic.
Hence, heat given to the gas in the left part is 0.
(c) There will be a common pressure ‘P‘ when equilibrium is reached. The slid will move until the pressure on the two sides becomes equal.
P1V1γ + P2V2γ = PV0γ
For equilibrium, V1 = V2 =
V02
Hence,
P1V02γ+P2V02γ=P(V0)γOr P=P11γ+P21γ2γ
Question 31:
An adiabatic cylindrical tube of cross-sectional area 1 cm2 is closed at one end and fitted with a piston at the other end. The tube contains 0.03 g of an ideal gas. At 1 atm pressure and at the temperature of the surrounding, the length of the gas column is 40 cm. The piston is suddenly pulled out to double the length of the column. The pressure of the gas falls to 0.355 atm. Find the speed of sound in the gas at atmospheric temperature.
Answer:
Given:
Area of the tube, A = 1 cm2 = 1 × 10−4 m2
Mass of the gas, M = 0.03 g = 0.03 × 10−3 kg
Initial pressure, P = 1 atm = 105 Pascal
Initial length of the mercury column, L = 40 cm = 0.4 m
Final length of the mercury column, L1 = 80 cm = 0.8 m
Final pressure, P’ = 0.355 atm
The process is adiabatic. So,
P(V)γ = P’(V’)γ
⇒ 1 × (A × 0.4)γ = 0.355 × (A × 0.8)γ
⇒ 1 × 1 = 0.355 × 2γ
⇒10.355=2γγ log 2=log10.355⇒ γ = 1.4941
Speed of sound in the gas,
v=γPρ=γPm/vv=1.4941×105m/v=1.4941×1050.03×10-310-4×1×0.4⇒v=446.33≈447 m/s
Page No 80:
Question 32:
The speed of sound in hydrogen at 0°C is 1280 m s−1. The density of hydrogen at STP is 0.089 kg m−3. Calculate the molar heat capacities Cp and Cv of hydrogen.
Answer:
Given:
Velocity of sound in hydrogen, V = 1280 m/s
Temperature, T = 0°C = 273 K
Density of H2 = 0.089 kg/m3
R = 8.3 J/mol-K
At STP,
P = 105 Pa
We know:
Vsound=γpρ1280=γ×1050.089Or γ=1280×1280×0.089105 =1.46CpCv=γ or Cp-Cv=RCv=Rγ-1=8.31.46-1 =18.0 J/mol-KCp=γCv=1.46×18.0 =26.28 ≈26.3/mol-K
Question 33:
4.0 g of helium occupies 22400 cm3 at STP. The specific heat capacity of helium at constant pressure is 5.0 cal K−1 mol−1. Calculate the speed of sound in helium at STP.
Answer:
Given:
Specific heat capacity at constant pressure, Cp = 5.0 cal/mol-K
Cp = 5.0 × 4.2 J/mol-K
Cp = 21 J/mol-K
Volume of helium, V = 22400
cm3= 0.0224
m3At STP, P = 1 atm =
105 PaThe speed of sound in gas,
v=γpρ=γRTM=γPVMCp=Rγγ-1Or 21(γ − 1) = 8.3γ
⇒ 21γ − 8.3γ = 21
⇒ 12.7γ = 21
∴ γ=2112.7=1.653v=1.653×1.0×105×0.02244×10-3v=960 m/s
Question 34:
An ideal gas of density 1.7 × 10−3 g cm−3 at a pressure of 1.5 × 105 Pa is filled in a Kundt’s tube. When the gas is resonated at a frequency of 3.0 kHz, nodes are formed at a separation of 6.0 cm. Calculate the molar heat capacities Cp and Cv of the gas.
Answer:
Given:
Density of the ideal gas, ρ = 1.7 × 10−3 g/cm3
= 1.7 k/gm3
Pressure of the gas, P = 1.5 × 105 Pa
R = 8.3 J/mol-K
Resonance frequency of the gas = 3.0 kHz
Node separation in the Kundt’s tube
=
l2= 6 cm
So, l = 2
×6 = 12 cm = 12 × 10−2 m
So, V = fl = 3 × 103 × 12 × 10−2
= 360 m/s
Speed of sound, V =
γpρOr
V2=γpρ
∴ γ=v2ρP=(360)2 ×1.7×10-31.5×105 =1.4688Using Cp-Cv=R and CpCv=γWe know that Cv=Rγ-1=8.30.4688 =17.7 J/mol-KCp=R + Cv=8.3 + 17.7 = 26 J/mol-K
Question 35:
Standing waves of frequency 5.0 kHz are produced in a tube filled with oxygen at 300 K. The separation between the consecutive nodes is 3.3 cm. Calculate the specific heat capacities Cp and Cv of the gas.
Answer:
Frequency of standing waves, f = 5 × 103 Hz
Temperature of oxygen, T = 300 K
From Kundt’s tube theory, we know that
l2= node separation = 3.3 cm
∴ l = 6.6 × 10−2 m
Also,
v = fl = 5 × 103 × 6.6 × 10−2
= (66 × 5) = 330 m/s
v=γRTM v2=γRTM∴ γ=330×330×328.3×300×100=1.3995Specific heat at constant volume, Cv=Rγ-1=8.30.3995= 20.7 J mol-K
Specific heat at constant pressure, Cp = Cv + R
Cp = 20.7 + 8.3
Cp = 29.0 J/mol-K
Chapterwise HC Verma Solutions Class 12 Physics :
- Chapter 23 – Heat and Temperature
- Chapter 24 – Kinetic Theory of Gases
- Chapter 25 – Calorimetry
- Chapter 26 – Laws of Thermodynamics
- Chapter 27 – Specific Heat Capacities of Gases
- Chapter 28 – Heat Transfer
- Chapter 29 – Electric Field and Potential
- Chapter 30 – Gauss’s Law
- Chapter 31 – Capacitors
- Chapter 32 – Electric Current in Conductors
- Chapter 33 – Thermal and Chemical Effects of Electric Current
- Chapter 34 – Magnetic Field
- Chapter 35 – Magnetic Field due to a Current
- Chapter 36 – Permanent Magnets
- Chapter 37 – Magnetic Properties of Matter
- Chapter 38 – Electromagnetic Induction
- Chapter 39 – Alternating Current
- Chapter 40 – Electromagnetic Waves
- Chapter 41 – Electric Current through Gases
- Chapter 42 – Photoelectric Effect and Wave Particle Duality
- Chapter 43 – Bohr’s Model and Physics of the Atom
- Chapter 44 – X-rays
- Chapter 45 – Semiconductors and Semiconductor Devices
- Chapter 46 – The Nucleus
- Chapter 47 – The Special Theory of Relativity
About the Author – HC Verma
HC Verma, the author of many popular and well-renowned Physics books, was born on 8 April 1952. Passing out from one of the most prestigious colleges of the country, IIT Kanpur, he worked as an experimental physicist in the Department of Nuclear Physics.
His most famous works which he is known for include the two-volume Concepts of Physics. He also worked for the social upliftment of the economically weaker children through his organization named Shiksha Sopan. He is also the recipient of the Padma Shri, which is considered India’s fourth-highest civilian award. He received the same because of his contribution and valuable work in the field of Physics.
